Abstract
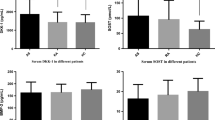
Ankylosing spondylitis (AS) patients whose symptom onset occurs before 16 years of age are termed juvenile-onset ankylosing spondylitis (JAS). Investigations suggested that JAS had worse functional outcome, and abnormality of bone metabolism can appear in early stage of AS. The objectives of this study are to compare changes of serum inflammatory and bone metabolic markers and to explore the relationship between these biomarkers and disease activity in JAS with different HLA-B27 subtypes. Serum matrix metallopeptidase-3 (MMP-3), soluble receptor activator of nuclear factor-κB ligand (sRANKL), and osteoprotegerin (OPG) were detected by ELISA in 56, 62, and 68 JAS patients, respectively, and 32 healthy individuals were as controls. Serum MMP-3 and sRANKL were significantly higher and OPG in JAS was slightly higher than those in controls. There was no significant difference in the level of MMP-3, sRANKL, and OPG among JAS patients with B27 negativity, B*2704, B*2705, and B*2715, respectively. Serum levels of MMP-3 showed positive correlation with BASDAI and BASFI (Bath Ankylosing Spondylitis Disease Activity Index and Functional Index). Serum level of sRANKL showed positive correlation with MMP-3 and negative correlation with disease duration. The significantly higher sRANKL expression suggested the enhanced osteoclast function and imbalance of RANKL/OPG system in the inflammatory process of JAS patients carrying different B27 subtypes. It should be paid attention to the abnormality of bone metabolism during the treatment of JAS.
Similar content being viewed by others
References
Shi GY (1999) Early clinical characteristic of juvenile-onset spondyloarthropathies. Chin J Rheumatol 3(3):131–132
Lin YC, Liang TH, Chen WS et al (2009) Differences between juvenile-onset ankylosing spondylitis and adult-onset ankylosing spondylitis. J Chin Med Assoc 72(22):573–580
Stone M, Warren RW, Bruckel J et al (2005) Juvenile-onset ankylosing spondylitis is associated with worse functional outcomes than adult-onset ankylosing spondylitis. Arthritis Rheum 53(3):445–451
van der Weijden MA, van Denderen JC, Lems WF et al (2011) Low bone mineral density is related to male gender and decreased functional capacity in early spondylarthropathies. Clin Rheumatol 30(4):497–503
Bao J, Chen Y, Bao YX (2014) Prevalence and risk factors of low bone mineral density in juvenile onset ankylosing spondylitis. Calcif Tissue Int 95(2):108–111
Chen CH, Lin KC, Yu DT et al (2006) Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology 45:414–420
Viswanath V, Myles A, Dayal R et al (2011) Levels of serum matrix metalloproteinase-3 correlate with disease activity in the enthesitis-related arthritis category of juvenile idiopathic arthritis. J Rheumatol 38(11):2482–2487
Maksymowych WP, Landewé R, Conner-Spady B et al (2007) Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum 56(6):1846–1853
Vandooren B, Kruithof E, Yu DT et al (2004) Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and down-regulation by tumor necrosis factor alpha blockade in spondylarthropathy. Arthritis Rheum 50(9):2942–2953
Sarma PK, Misra R, Aggarwal A (2008) Elevated serum receptor activator of NF kappaB ligand (RANKL), osteoprotegerin (OPG), matrix metalloproteinase (MMP)3, and ProMMP1 in patients with juvenile idiopathic arthritis. Clin Rheumatol 27(3):289–294
Thor U, Arne Y, Øie E et al (2005) Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation 111:2461–2468
Sato K, Takayanagi H (2006) Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol 18:419–426
Boyce BF, Schwarz EM, Xing LP (2006) Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. CurrOpin Rheumatol 18:427–432
Lone J, John-Bjarne H, Jan B et al (2011) Serum osteoprotegerin levels are related to height loss: the Tromsø Study. Eur J Epidemiol 26:305–312
Crotti TN, Smith MD, Weedon H et al (2002) Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis 61:1047–1054
Haynes DR, Barg E, Crotti TN et al (2003) Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology 42:123–134
Wendling D (2005) Bone loss in AS: can we put the puzzle together? J Rheumatol 32:1184–1185
Lems W (2007) Clinical relevance of vertebral fractures: why are vertebral fractures so often overlooked? Ann Rheum Dis 66:2–4
Mou Y, Wu Z, Gu J, Liao Z et al (2010) HLA-B27 polymorphism in patients with juvenile and adult-onset ankylosing spondylitis in Southern China. Tissue Antigens 75:56–60
Jie-ruo GU, Han-wei ZHANF, Li-ke ZHAO et al (2004) The significance of matrix metalloproteinase-3 response down-regulation in the treatment of ankylosing spondylitis by anti-TNF-a monoclonal antibody remicade. Chin Rem Clin 4(4):270–276
Viswanath V, Myles A, Dayal R et al (2011) Levels of serum matrix metalloproteinase-3 correlate with disease activity in the enthesitis-related arthritis category of juvenile idiopathic arthritis. J Rheumatol 38(11):2482–2487
Takayanagi H, Iizuka H, Juji T et al (2000) Involvement of receptor activator of nuclear factorκB ligand osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43:259–269
Dayer JM, Bresnihan B (2002) Targeting interleukin 1 in the treatment of rheumatoid arthritis. Arthritis Rheum 46:574–578
Takayanagi H (2005) Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83(3):170–179
Ta HM, Nguyen GT, Jin HM et al (2010) Structure-based development of a receptor activator of nuclear factor-kappaB ligand (RANKL) inhibitor peptide and molecular basis for osteopetrosis. Proc Natl Acad Sci U S A 107(47):20281–20286
Grazio S, Kusić Z, Cvijetić S et al (2012) Relationship of bone mineral density with disease activity and functional ability in patients with ankylosing spondylitis: a cross-sectional study. Rheumatol Int 32(9):2801–2808
Van der Weijden MA, van Denderen JC, Lems WF et al (2011) Low bone mineral density is related to male gender and decreased functional capacity in early spondylarthropathies. Clin Rheumatol 30(4):497–503
Arends S, Spoorenberg A, Bruyn GA et al (2011) The relation between bone mineral density, bone turnover markers, and vitamin D status in ankylosing spondylitis patients with active disease: a cross-sectional analysis. Osteoporos Int 22(5):1431–1439
Franck H, Meurer T, Hofbauer LC (2004) Evaluation of bone mineral density, hormones, biochemical markers of bone metabolism, and osteoprotegerin serum levels in patients with ankylosing spondylitis. J Rheumatol 31(11):2236–2241
Kim HR, Lee SH, Kim HY (2006) Elevated serum levels of soluble receptor activator of nuclear factors-kappaB ligand (sRANKL) and reduced bone mineral density in patients with ankylosing spondylitis (AS). Rheumatology 45:1197–1200
Téletchéa S, Stresing V, Hervouet S et al (2014) Novel RANK antagonists for the treatment of bone-resorptive disease: theoretical predictions and experimental validation. J Bone Miner Res 29(6):1466–77
Acknowledgments
This work was supported by the 5010 Subject of Sun Yat-sen University (2007023) and National Natural Science Foundation of China Grant (31070806).
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yi-Kun Mou and Ping-Ping Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mou, YK., Zhang, PP., Li, QX. et al. Changes of serum levels of MMP-3, sRANKL, and OPG in juvenile-onset ankylosing spondylitis patients carrying different HLA-B27 subtypes. Clin Rheumatol 34, 1085–1089 (2015). https://doi.org/10.1007/s10067-015-2940-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2940-z