Abstract
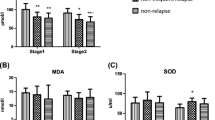
The aim of this study was to assess the role of oxidative stress in the pathogenesis of Henoch–Schönlein purpura (HSP) vasculitis. The activities of catalase (CAT), arylesterase (ARYL), and paraoxonase (PON) as antioxidant enzymes and serum malondialdehyde (MDA) level as an indicator of lipid peroxidation, together with total antioxidant status (TAS), were measured in 29 children with HSP (mean age 9.3 ± 2.7 years), both at the onset of the disease and at the remission period and in matched controls. Active-stage HSP had significantly higher MDA level (15.5 ± 7.3 vs 7.8 ± 3.9 nmol/l, respectively, P < 0.001) and lower TAS (524 ± 122 vs 699 ± 122 μmol Trolox Equiv/l, P < 0.001), PON (97 ± 47 vs 136 ± 95 U/l, P = 0.042), ARYL (158 ± 39 vs 212 ± 52 U/l, P < 0.001), and CAT (50 ± 27 vs 69 ± 20 U/l, P = 0.002) activities compared with the control subjects. Although CAT (P > 0.05) and PON (P > 0.05) activities were found to be similar between active and remission stages of HSP, the active stage of the disease had significantly lower ARYL (P = 0.011) and TAS (P = 0.006) and higher MDA (P < 0.001) values compared with remission period. Significant positive correlations were found between CAT and MDA (r = 0.433, P = 0.019) and between CAT and C-reactive protein (r = 0.386, P = 0.035) in the active stage of HSP. No significant differences were detected in oxidant/antioxidant parameters between patients with or without renal, gastrointestinal, or joint involvement (P > 0.05). Increased oxidative stress and lipid peroxidation may play important roles in the pathogenesis of HSP vasculitis. Antioxidant therapeutic interventions in long-lasting vasculitis and risk of atherosclerosis secondary to increased oxidant stress remain to be investigated.


Similar content being viewed by others
References
Buyan N, Erbas D, Akkok N et al (1998) Role of free oxygen radicals and prostanoids in the pathogenesis of Henoch–Schonlein Purpura. Prostaglandins Leukot Essent Fat Acids 59:181–184
McCord JM (1993) Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem 26:351–357
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721–724
Demircin G, Öner A, Ünver Y et al (1998) Erythrocyte superoxide dismutase activity and plasma malondialdehyde levels in children with Henoch–Schönlein purpura. Acta Pediatr 87:848–852
Erdoğan Ö, Öner A, Aydın A et al (2003) Effect of vitamin E treatment on the oxidative damage occurring in Henoch–Schönlein purpura. Acta Paediatr 92:546–550
Shah SV (1988) Evidence suggesting a role for hydroxyl radical in passive Heymann nephritis in rats. Am J Physiol 254:F337–F344
Rahman MA, Emancipator SS, Sedor JR (1988) Hydroxyl radical scavengers ameliorate proteinuria in rat immune complex glomerulonephritis. J Lab Clin Med 112:619–626
Turi S, Nemeth I, Torkos A et al (1997) Oxidative stress and antioxidant defense mechanism in glomerular diseases. Free Radic Biol Med 22:161–168
Cao G, Prior RL (1998) Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem 44:1309–1315
Rice-Evans C, Miller NJ (1994) Total antioxidant status in plasma and body fluids. Methods Enzymol 234:279–293
Janero DR (1990) Malondialdehyde and thiobarbituric-acid reactivity as diagnostic indices of lipid peroxidation, and peroxidative tissue injury. Free Radic Biol Med 9:515–540
Nielsen F, Mikkelsen BB, Nielsen JB et al (1997) Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem 43:1209–1214
Aviram M, Rosenblat M, Billecke S et al (1999) Human serum paraoxonase (PON) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med 26:892–904
Aviram M, Rosenblat M (2005) Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol 16:393–399
Mills JA, Michel BA, Bloch DA et al (1990) The American College of Rheumatology 1990 criteria for the classification of Henoch–Schönlein purpura. Arthritis Rheum 33:1114–1121
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chem Acta 196:143–151
Furlong CE, Richter RJ, Seidel SL et al (1989) Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal Biochem 180:242–247
Haagen L, Brock A (1992) A new automated method for phenotyping arylesterase (E.C.3·1·1·2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 30:391–395
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37:112–119
Asakawa T, Matsushita S (1980) Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 15:137–141
Al-Sheyyab M, El-Shanti H, Ajlouni S et al (1996) Henoch–Schönlein purpura: clinical experience and contemplation on a streptococcal association. J Trop Pediatr 42:200–203
Freeman BA, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Invest 47:412–426
Minuz P, Fava C, Cominacini L (2006) Oxidative stress, antioxidants, and vascular damage. Br J Clin Pharmacol 61:774–777
Öner A, Tınaztepe K, Erdoğan Ö (1995) The effect of triple therapy on rapidly progressive type of Henoch–Schönlein nephritis. Pediatr Nephrol 9:6–10
Iuchi T, Akaike M, Mitsui T et al (2003) Glucorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res 92:81–87
Ece A, Atamer Y, Gürkan F et al (2005) Paraoxonase, total antioxidant response, and peroxide levels in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 20:1279–1284
Harper L, Nuttall SL, Martin U, Savage CO (2002) Adjuvant treatment of patients with antineutrophil cytoplasmic antibody-associated vasculitis with vitamins E and C reduces superoxide production by neutrophils. Rheumatology (Oxford) 41:274–278
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ece, A., Kelekçi, S., Kocamaz, H. et al. Antioxidant enzyme activities, lipid peroxidation, and total antioxidant status in children with Henoch–Schönlein purpura. Clin Rheumatol 27, 163–169 (2008). https://doi.org/10.1007/s10067-007-0671-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0671-5