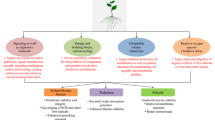
Abstract
The interaction between the dual roles of sugar as a metabolic fuel and a regulatory molecule was unveiled by examining the changes in sugar signaling upon oxygen deprivation, which causes the drastic alteration in the cellular energy status. In our study, the expression of anaerobically induced genes is commonly responsive to sugar, either under the control of hexokinase or non-hexokinase mediated signaling cascades. Only sugar regulation via the hexokinase pathway was susceptible for O2 deficiency or energy deficit conditions evoked by uncoupler. Examination of sugar regulation of those genes under anaerobic conditions revealed the presence of multiple paths underlying anaerobic induction of gene expression in rice, subgrouped into three distinct types. The first of these, which was found in type-1 genes, involved neither sugar regulation nor additional anaerobic induction under anoxia, indicating that anoxic induction is a simple result from the release of sugar repression by O2-deficient conditions. In contrast, type-2 genes also showed no sugar regulation, albeit with enhanced expression under anoxia. Lastly, expression of type-3 genes is highly enhanced with sugar regulation sustained under anoxia. Intriguingly, the inhibition of the mitochondrial ATP synthesis can reproduce expression pattern of a specific set of anaerobically induced genes, implying that rice cells may sense O2 deprivation, partly via perception of the perturbed cellular energy status. Our study of interaction between sugar signaling and anaerobic conditions has revealed that sugar signaling and the cellular energy status are likely to communicate with each other and influence anaerobic induction of gene expression in rice.
Similar content being viewed by others
References
Baena-Gonzalez, E. (2010). Energy signaling in the regulation of gene expression during stress. Mol. Plant 3, 300–313.
Baena-Gonzalez, E., and Sheen, J. (2008). Convergent energy and stress signaling. Trends Plant Sci. 13, 474–482.
Baena-Gonzalez, E., Rolland, F., Thevelein, J.M., and Sheen, J. (2007). A central integrator of transcription networks in plant stress and energy signaling. Nature 448, 938–942.
Cho, J.I., Ryoo, N., Eom, J.S., Lee, D.W., Kim, H.B., Jeong, S.W., Lee, Y.H., Kwon, Y.K., Cho, M.H., Bhoo, S.H., et al. (2009a). Role of the rice hexokinases OsHXK5 and OsHXK6 as glucose sensors. Plant Physiol. 149, 745–759.
Cho, J.I., Ryoo, N., Hahn, T.R., and Jeon, J.S. (2009b). Evidence for a role of hexokinases as conserved glucose sensors in both monocot and dicot plant species. Plant Signal. Behav. 4, 908–910.
Beevers, H. (1961). Respiratory metabolism in plants (New York: Row-Peterson and Company).
Fennoy, S.L., Jayachandran, S., and Bailey-Serres, J. (1997). RNase activities are reduced concomitantly with conservation of total cellular RNA and ribosomes in O2-deprived seedling roots of maize. Plant Physiol. 115, 1109–1117.
Gibbs, D.J., Lee, S.C., Isa, N.M., Gramuglia, S., Fukao, T., Bassel, G.W., Correia, C.S., Corbineau, F., Theodoulou, F.L., Bailey-Serres, J., et al. (2011). Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479, 415–418.
Green, P.J. (1993). Control of mRNA stability in higher plants. Plant Physiol. 102, 1065–1070.
Greenway, H., and Gibbs, J. (2003). Review: mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 30, 999–1036.
Hanson, J., and Smeekens, S. (2009). Sugar perception and signaling—an update. Curr. Opin. Plant Biol. 12, 562–567.
Hardie, D.G. (2007). AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785.
Hedbacker, K., and Carlson, M. (2008). SNF1/AMPK pathways in yeast. Front. Biosci. 13, 2408–2420.
Ho, S.L., Chao, Y.C., Tong, W.W., and Yu, S.M. (2001). Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control cechanisms. Plant Physiol. 125, 877–890.
Hoeren, F.U., Dolferus, R., Wu, Y., Peacock, W.J., and Dennis, E.S. (1998). Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics 149, 479–490.
Huang, N., Chandler, J., Thomas, B.R., Koizumi, N., and Rodriguez, R.L. (1993). Metabolic regulation of alpha-amylase gene expression in transgenic cell cultures of rice (Oryza sativa L.). Plant Mol. Biol. 23, 737–747.
Jang, J.C., Leon, P., Zhou, L., and Sheen, J. (1997). Hexokinase as a sugar sensor in higher plants. Plant Cell 9, 5–19.
Koch, K., Ying, Z., Wu, Y., and Avigne, W. (2000). Multiple paths of sugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolism. J. Exp. Bot. 51, 417–427.
Lasanthi-Kudahettige, R., Magneschi, L., Loreti, E., Gonzali, S., Licausi, F., Novi, G., Beretta, O., Vitulli, F., Alpi, A., and Perata, P. (2007). Transcript profiling of the anoxic rice coleoptile. Plant Physiol. 144, 218–231.
Lee, K.W., Chen, P.W., Lu, C.A., Chen, S., Ho, T.H., and Yu, S.M. (2009). Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci. Signal. 2, ra61.
Licausi, F., Kosmacz, M., Weits, D.A., Giuntoli, B., Giorgi, F.M., Voesenek, L.A., Perata, P., and van Dongen, J.T. (2011). Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479, 419–422.
Liu, F., Van Toai, T., Moy, L.P., Bock, G., Linford, L.D., and Quackenbush, J. (2005). Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Cell 137, 1115–1129.
Loughman, B.C., Ratcliffe, R.G., and Southon, T.E. (1989). Observations on the cytoplasmic and vacuolar orthophosphate pools in leaf tissues using in vivo 31P-NMR spectroscopy. FEBS Lett. 242, 279–284.
Lu, C.A., Lin, C.C., Lee, K.W., Chen, J.L., Huang, L.F., Ho, S.L., Liu, H.J., Hsing, Y.I., and Yu, S.M. (2007). The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. Plant Cell 19, 2484–2499.
McGee, S.L., and Hargreaves, M. (2008). AMPK and transcriptional regulation. Front. Biosci. 13, 3022–3033.
Mohanty, B., Herath, V., Wijaya, E., Yeo, H.C., de Los Reyes, B.G., and Lee, D.Y. (2012). Patterns of cis-element enrichment reveal potential regulatory modules involved in the transcriptional regulation of anoxia response of japonica rice. Gene 511, 235–242.
Mustroph, A., Boamfa, E.I., Laarhoven, L.J., Harren, F.J., Albrecht, G., and Grimm, B. (2006). Organ-specific analysis of the anaerobic primary metabolism in rice and wheat seedlings. I: Dark ethanol production is dominated by the shoots. Planta 225, 103–114.
Olive, M.R., Peacock, W.J., and Dennis, E.S. (1991). The anaerobic responsive element contains two GC-rich sequences essential for binding a nuclear protein and hypoxic activation of the maize Adh1 promoter. Nucleic Acids Res. 19, 7053–7060.
Park, M., Yim, H.K., Park, H.G., Lim, J., Kim, S.H., and Hwang, Y.S. (2010). Interference with oxidative phosphorylation enhances anoxic expression of rice alpha-amylase genes through abolishing sugar regulation. J. Exp. Bot. 61, 3235–3244.
Price, J., Laxmi, A., St. Martin, S.K., and Jang, J.C. (2004). Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16, 2128–2150.
Ramon, M., Rolland, F., and Sheen, J. (2008). Sugar sensing and signaling. The Arabidopsis book/American Society of Plant Biologists 6, e0117.
Raymond, P., Al-Ani, A., and Pradet, A. (1985). ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiol. 79, 879–884.
Rolland, F., Baena-Gonzalez, E., and Sheen, J. (2006). Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann. Rev. Plant Biol. 57, 675–709.
Salas, J., Salas, M., Vinuela, E., and Sols, A. (1965). Gucokinase of rabbit liver. J. Biol. Chem. 240, 1014–1018.
Sasidharan, R., and Mustroph, A. (2011). Plant oxygen sensing is mediated by the N-end rule pathway: a milestone in plant anaerobiosis. Plant Cell 23, 4173–4183.
Sheen, J. (2010). Discover and connect cellular signaling. Plant Physiol. 154, 562–566.
Sheu-Hwa, C.S., Lewis, D.H., and Waker, D.A. (1975). Stimulation of photosynthetic starch formation by sequestration of cytoplasmic orthophosphate. New Phytol. 74, 383–392.
Thompson, J., Abdullah, R., and Cocking, E. (1986). Protoplast culture of rice (Oryza sativa L.) using media solidified with agarose. Plant Sci. 47, 123–133.
Wang, H.J., Wan, A.R., Hsu, C.M., Lee, K.W., Yu, S.M., and Jauh, K.W. (2007). Transcriptomic adaptations in rice suspension cells under sucrose starvation. Plant Mol. Biol. 63, 441–463.
Wilson, C. (1975). Plant nucleases. Annu. Rev. Plant Biol. 26, 187–208.
Yim, H.K., Lim, M.N., Lee, S.E., Lim, J., Lee, Y., and Hwang, Y.S. (2012). Hexokinase-mediated sugar signaling controls expression of the calcineurin B-like interacting protein kinase 15 gene and is perturbed by oxidative phosphorylation inhibition. J. Plant Physiol. 169, 1551–1558.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lim, Mn., Lee, Se., Yim, Hk. et al. Differential anoxic expression of sugar-regulated genes reveals diverse interactions between sugar and anaerobic signaling systems in rice. Mol Cells 36, 169–176 (2013). https://doi.org/10.1007/s10059-013-0152-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-013-0152-4