Abstract
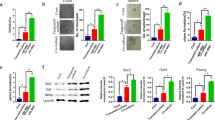
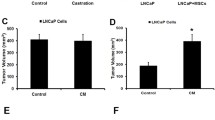
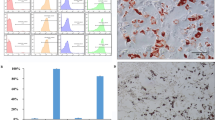
Although cancer stem cells (CSCs) play a crucial role in seeding the initiation of tumor progression, they do not always possess the same potent ability as tumor metastasis. Thus, precisely how migrating CSCs occur, still remains unclear. In the present study, we first comparatively analyzed a series of prostate CSCs, which exhibited a dynamically increasing and disseminating ability in nude mice. We observed that the transcriptional activity of HIF-1α and β-catenin became gradually elevated in these stem cells and their epithelial-mesenchymal transition (EMT) characteristic altered from an epithelial type to a mesenchymal type. Next, we further used cancer-associated fibroblasts (CAFs), which were cultured from surgically resected tissues of prostate cancer (PCa) to stimulate prostate CSCs. Similar results were reconfirmed and showed that the protein levels of both HIF-1α and β-catenin were markedly improved. In addition, the EMT phenotype displayed a homogenous mesenchymal type, accompanied with increased aggressive potency in vitro. Most importantly, the aforementioned promoting effect of CAFs on prostate CSCs was completely repressed after “silencing” the activity of β-catenin by transfection of stem cells with ShRNA. Taken together, our observations suggest that prostate migrating CSCs, with a mesenchymal phenotype, could be triggered by CAFs in a HIF-1α/β-catenin-dependent signaling pathway.
Similar content being viewed by others
References
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F., and Kirchner, T. (2005). Opinion: migrating cancer stem cells — an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749.
Cat, B., Stuhlmann, D., Steinbrenner, H., Alili, L., Holtkötter, O., Sies, H., and Brenneisen, P. (2006). Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J. Cell Sci. 119, 2727–2738.
Chung, L.W., Baseman, A., Assikis, V., and Zhau, H.E. (2005). Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J. Urol. 173, 10–20.
Conley, S.J., Gheordunescu, E., Kakarala, P., Newman, B., Korkaya, H., Heath, A.N., Clouthier, S.G., and Wicha, M.S. (2012). Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Natl. Acad. Sci. USA 109, 2784–2789.
De Wever, O., and Mareel, M. (2003). Role of tissue stroma in cancer cell invasion. J. Pathol. 200, 429–447.
Fidler, I.J. (2003). The pathogenesis of cancer metastasis: the’ seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458.
Giannoni, E., Bianchini, F., Masieri, L., Serni, S., Torre, E., Calorini, L., and Chiarugi, P. (2010). Reciprocal activation of prostate cancer cells and cancerassociated fibroblasts stimulates epithelialmesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956.
Giannoni, E., Bianchini, F., Calorini, L., and Chiarugi, P. (2011). Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 14, 2361–2371.
Hermann, P.C., Huber, S.L., Herrler, T., Aicher, A., Ellwart, J.W., Guba, M., Bruns, C.J., and Heeschen, C. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1, 313–323.
Hwang, R.F., Moore, T., Arumugam, T., Ramachandran, V., Amos, K.D., Rivera, A., Ji, B., Evans, D.B., and Logsdon, C.D. (2008). Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926.
Jiang, Y.G., Luo, Y., He, D.L., Li, X., Zhang, L.L., Peng, T., Li, M.C., and Lin, Y.H. (2007). Role of Wnt/beta-catenin signaling path way in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int. J. Urol. 14, 1034–1039.
Joyce, J.A., and Pollard, J.W. (2009). Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252.
Kalluri, R. (2009). EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 119, 1417–1419.
Kalluri, R., and Zeisberg, M. (2006). Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401.
Kaminski, A., Hahne, J.C., Haddouti, E., Florin, A., Wellmann, A., and Wernert, N. (2006). Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int. J. Mol. Med. 18, 941–950.
Klarmann, G.J., Hurt, E.M., Mathews, L.A., Zhang, X., Duhagon, M.A., Mistree, T., Thomas, S.B., and Farrar, W.L. (2009). Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis 26, 433–446.
Lebret, S.C., Newgreen, D.F., Thompson, E.W., and Ackland, M.L. (2007). Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res. 9, R19.
Liao, C.P., Adisetiyo, H., Liang, M., and Roy-Burman, P. (2010). Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 70, 7294–7303.
Liotta, L.A., and Kohn, E.C. (2001). The microenvironment of the tumour-host interface. Nature 411, 375–379.
Luo, Y., He, D.L., Ning, L., Shen, S.L., Li, L., Li, X., Zhau, H.E., and Chung, L.W. (2006). Over-expression of hypoxia-inducible factor-1alpha increases the invasive potency of LNCaP cells in vitro. BJU Int. 98, 1315–1319.
Luo, Y., Cui, X.H., Jiang, Y.G., Zhao, J.H., Zhao, L., Chen, Y.T., Li, M.C., and Lin, Y.H. (2012). Sorting and identification of cancer stem cells in human prostate cancer cell lines. Zhonghua Nan Ke Xue 18, 1062–1068.
Luo, Y., Cui, X.H., Jiang, Y.G., He, D.L., Zhao, J.H., Zhao, L., Chen, Y.T., Li, M.C., and Lin, Y.H. (2013). Epithelial-mesenchymal transition of prostate cancer: cancer stem cells or bulk cancer cells. Zhonghua Yi Xue Za Zhi 93, 256–260.
Mani, S.A., Guo, W., Liao, M.J., Eaton, E.N., Ayyanan, A., Zhou, A.Y., Brooks, M., Reinhard, F., Zhang, C.C., Shipitsin, M., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715.
Silzle, T., Randolph, G.J., Kreutz, M., and Kunz-Schughart, L.A. (2004). The fibroblast: sentinel cell and local immune modulator in tumor tissue. Int. J. Cancer 108, 173–180.
Studebaker, A.W., Storci, G., Werbeck, J.L., Sansone, P., Sasser, A.K., Tavolari, S., Huang, T., Chan, M.W., Marini, F.C., Rosol, T.J., et al. (2008). Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 68, 9087–9095.
Sung, S.Y., Hsieh, C.L., Law, A., Zhau, H.E., Pathak, S., Multani, A.S., Lim, S., Coleman, I.M., Wu, L.C., Figg, W.D., et al. (2008). Coevolution of prostate cancer and bone stroma in threedimensional coculture: implications for cancer growth and metastasis. Cancer Res. 68, 9996–10003.
Thiery, J.P., Acloque, H., Huang, R.Y., and Nieto, M.A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890.
Visvader, J.E., and Lindeman, G.J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768.
Yang, Z.F., Ho, D.W., Ng, M.N., Lau, C.K., Yu, W.C., Ngai, P., Chu, P.W., Lam, C.T., Poon, R.T., and Fan, S.T. (2008). Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13, 153–166.
Zhao, J.H., Luo, Y., Jiang, Y.G., He, D.L., and Wu, C.T. (2011). Knockdown of β-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1α. Cancer Invest. 29, 377–382.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Luo, Y., Lan, L., Jiang, YG. et al. Epithelial-mesenchymal transition and migration of prostate cancer stem cells is driven by cancer-associated fibroblasts in an HIF-1α/β-catenin-dependent pathway. Mol Cells 36, 138–144 (2013). https://doi.org/10.1007/s10059-013-0096-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-013-0096-8