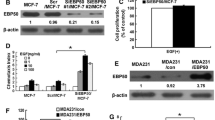
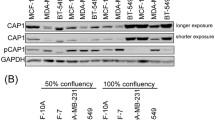
Abstract
The actin cytoskeleton plays an important role in cell shape determination, adhesion and cell cycle progression. Ezrinradixin-moesin (ERM)-binding phosphoprotein 50 (EBP50), also known as Na+-H+ exchanger regulatory factor 1 (NHERF1), associates with actin cytoskeleton and is related to cell cycle progression. Its Ser279 and Ser301 residues are phosphorylated by cyclin-dependent kinase 2 (cdc2)/cyclin B during the mitosis phase. However, the biological significance of EBP50 phosphorylation mediated by cdc2/cyclin B is not clear. In the present study, MDA-MB-231 cells with low levels of endogenous EBP50 protein were stably transfected with constructs of EBP50 wild type (WT), phosphodeficient (serine 279 and serine 301 mutated to alanine-S279A/S301A) or phospho-mimetic (serine 279 and serine 301 mutated to aspartic acid-S279D/S301D) mutants. Subsequently, multiple phenotypes of these cells were characterized. Failure of cdc2/cyclin B-mediated EBP50 phosphorylation in cells expressing S279A/S301A (AA cells) significantly increased F-actin content, enhanced the adherence of cells to the extracellular matrix, altered cell morphology and caused defects in cytokinesis, as reflected in the formation of giant cells with heteroploid DNA and multinucleation or giant nuclei. Furthermore, knockdown of EBP50 expression in AA cells rescued cell defects such as the cytokinesis failure and abnormal cell morphology. EBP50 S279A/ S301A had a weaker binding affinity with actin than EBP50 S279D/S301D, which might explain the increase of F-actin content in the AA cells. The present results suggest that cdc2/cyclin B-mediated EBP50 phosphorylation may play a role in the regulation of various cell functions by affecting actin cytoskeleton reorganization.
Similar content being viewed by others
References
Baatout, S., Chatelain, B., Staquet, P., Symann, M., and Chatelain, C. (1998). Induction and enhancement of normal human megakaryocyte polyploidization are concomitant with perturbation in the actin metabolism. Eur. J. Clin. Invest. 28, 845–855.
Bai, R., Verdier-Pinard, P., Gangwar, S., Stessman, C.C., McClure, K.J., Sausville, E.A., Pettit, G.R., Bates, R.B., and Hamel, E. (2001). Dolastatin 11, a marine depsipeptide, arrests cells at cytokinesis and induces hyperpolymerization of purified actin. Mol. Pharmacol. 59, 462–469.
Bramwell, V.H., Crowther, D., Gallagher, J., and Stoddart, R.W. (1982). Studies of lectin binding to normal and neoplastic lymphoid tissues. I. Normal nodes and Hodgkin’s disease. Br J. Cancer 46, 568–581.
Bretscher, A. (1991). Microfilament structure and function in the cortical cytoskeleton. Annu. Rev. Cell Biol. 7, 337–374.
Bretscher, A., Reczek, D., and Berryman, M. (1997). Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 110, 3011–3018.
Castillo-Romero, A., Leon-Avila, G., Perez Rangel, A., Cortes Zarate, R., Garcia Tovar, C., and Hernandez, J.M. (2009). Participation of actin on Giardia lamblia growth and encystation. PLoS One 4, e7156.
Chang, E., Heo, K.S., Woo, C.H., Lee, H., Le, N.T., Thomas, T.N., Fujiwara, K., and Abe, J. (2010). MK2 SUMOylation regulates actin filament remodeling and subsequent migration in endothelial cells by inhibiting MK2 kinase and HSP27 phosphorylation. Blood 117, 2527–2537.
Cheng, H., Li, J., Fazlieva, R., Dai, Z., Bu, Z., and Roder, H. (2009). Autoinhibitory interactions between the PDZ2 and C-terminal domains in the scaffolding protein NHERF1. Structure 17, 660–669.
Dai, J.L., Wang, L., Sahin, A.A., Broemeling, L.D., Schutte, M., and Pan, Y. (2004). NHERF (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene 23, 8681–8687.
Demoulin, J.B., Seo, J.K., Ekman, S., Grapengiesser, E., Hellman, U., Ronnstrand, L., and Heldin, C.H. (2003). Ligand-induced recruitment of Na+/H+-exchanger regulatory factor to the PDGF (platelet-derived growth factor) receptor regulates actin cytoskeleton reorganization by PDGF. Biochem. J. 376, 505–510.
Gisler, S.M., Stagljar, I., Traebert, M., Bacic, D., Biber, J., and Murer, H. (2001). Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J. Biol. Chem. 276, 9206–9213.
Grzanka, D., Marszalek, A., Gagat, M., Izdebska, M., Gackowska, L., and Grzanka, A. (2010). Doxorubicin-induced F-actin reorganization in cofilin-1 (nonmuscle) down-regulated CHO AA8 cells. Folia Histochem. Cytobiol. 48, 377–386.
Hable, W.E., Miller, N.R., and Kropf, D.L. (2003). Polarity establishment requires dynamic actin in fucoid zygotes. Protoplasma 221, 193–204.
He, J., Lau, A.G., Yaffe, M.B., and Hall, R.A. (2001). Phosphorylation and cell cycle-dependent regulation of Na+/H+ exchanger regulatory factor-1 by Cdc2 kinase. J. Biol. Chem. 276, 41559–41565.
Hotulainen, P., Paunola, E., Vartiainen, M.K., and Lappalainen, P. (2005). Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell. 16, 649–664.
Hsu, F.F., Lin, T.Y., Chen, J.Y., and Shieh, S.Y. (2010). p53-Mediated transactivation of LIMK2b links actin dynamics to cell cycle checkpoint control. Oncogene 29, 2864–2876.
Hu, S., Biben, T., Wang, X., Jurdic, P., and Geminard, J.C. (2010). Internal dynamics of actin structures involved in the cell motility and adhesion: modeling of the podosomes at the molecular level. J. Theor. Biol. 270, 25–30.
Kunda, P., and Baum, B. (2009). The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 19, 174–179.
Lee, G.H., Ahn, T., Kim, D.S., Park, S.J., Lee, Y.C., Yoo, W.H., Jung, S.J., Yang, J.S., Kim, S., Muhlrad, A., et al. (2010). Bax inhibitor 1 increases cell adhesion through actin polymerization: involvement of calcium and actin binding. Mol. Cell. Biol. 30, 1800–1813.
Li, J., Poulikakos, P.I., Dai, Z., Testa, J.R., Callaway, D.J., and Bu, Z. (2007). Protein kinase C phosphorylation disrupts Na+/H+ exchanger regulatory factor 1 autoinhibition and promotes cystic fibrosis transmembrane conductance regulator macromolecular assembly. J. Biol. Chem. 282, 27086–27099.
Moh, M.C., Tian, Q., Zhang, T., Lee, L.H., and Shen, S. (2009). The immunoglobulin-like cell adhesion molecule hepaCAM modulates cell adhesion and motility through direct interaction with the actin cytoskeleton. J. Cell Physiol. 219, 382–391.
Mohler, P.J., Kreda, S.M., Boucher, R.C., Sudol, M., Stutts, M.J., and Milgram, S.L. (1999). Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J. Cell Biol. 147, 879–890.
Morales, F.C., Takahashi, Y., Kreimann, E.L., and Georgescu, M.M. (2004). Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci. USA 101, 17705–17710.
Moulding, D.A., Blundell, M.P., Spiller, D.G., White, M.R., Cory, G. O., Calle, Y., Kempski, H., Sinclair, J., Ancliff, P.J., Kinnon, C., et al. (2007). Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J. Exp. Med. 204, 2213–2224.
Murphy-Ullrich, J.E. (2001). The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J. Clin. Invest. 107, 785–790.
Murthy, A., Gonzalez-Agosti, C., Cordero, E., Pinney, D., Candia, C., Solomon, F., Gusella, J., and Ramesh, V. (1998). NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J. Biol. Chem. 273, 1273–1276.
Nestor, M.W., Cai, X., Stone, M.R., Bloch, R.J., and Thompson, S.M. (2011). The actin binding domain of betaI-spectrin regulates the morphological and functional dynamics of dendritic spines. PLoS One 6, e16197.
Pan, Y., Wang, L., and Dai, J.L. (2006). Suppression of breast cancer cell growth by Na+/H+ exchanger regulatory factor 1 (NHERF1). Breast Cancer Res. 8, R63.
Pollard, T.D., and Cooper, J.A. (2009). Actin, a central player in cell shape and movement. Science 326, 1208–1212.
Raghuram, V., Hormuth, H., and Foskett, J.K. (2003). A kinaseregulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc. Natl. Acad. Sci. USA 100, 9620–9625.
Reczek, D., Berryman, M., and Bretscher, A. (1997). Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139, 169–179.
Shao, H., Wang, J.H., Pollak, M.R., and Wells, A. (2010). α-actinin-4 is essential for maintaining the spreading, motility and contractility of fibroblasts. PLoS One 5, e13921.
Shenolikar, S., Voltz, J.W., Cunningham, R., and Weinman, E.J. (2004). Regulation of ion transport by the NHERF family of PDZ proteins. Physiology 19, 362–369.
Short, D.B., Trotter, K.W., Reczek, D., Kreda, S.M., Bretscher, A., Boucher, R.C., Stutts, M.J., and Milgram, S.L. (1998). An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273, 19797–19801.
Sun, F., Hug, M.J., Lewarchik, C.M., Yun, C.H., Bradbury, N.A., and Frizzell, R.A. (2000). E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J. Biol. Chem. 275, 29539–29546.
Voltz, J.W., Brush, M., Sikes, S., Steplock, D., Weinman, E.J., and Shenolikar, S. (2007). Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J. Biol. Chem. 282, 33879–33887.
Watabe, S., Wada, S., Saito, S., Matsunaga, S., Fusetani, N., Ozaki, H., and Karaki, H. (1996). Cellular changes of rat embryonic fibroblasts by an actin-polymerization inhibitor, bistheonellide A, from a marine sponge. Cell Struct. Funct. 21, 199–212.
White, J.G. (1984). Arrangements of actin filaments in the cytoskeleton of human platelets. Am. J. Pathol. 117, 207–217.
White, S.R., Williams, P., Wojcik, K.R., Sun, S., Hiemstra, P.S., Rabe, K.F., and Dorscheid, D.R. (2001). Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 24, 282–294.
Wong, G.K., Allen, P.G., and Begg, D.A. (1997). Dynamics of filamentous actin organization in the sea urchin egg cortex during early cleavage divisions: implications for the mechanism of cytokinesis. Cell Motil. Cytoskeleton 36, 30–42.
Yamagishi, T., and Kawai, H. (2011). Cytoskeleton organization during the cell cycle in two stramenopile microalgae, Ochromonas danica (Chrysophyceae) and Heterosigma akashiwo (Raphidophyceae), with special reference to F-actin organization and its role in cytokinesis. Protist 163, 686–700.
Yang, L., Wang, Y., Chen, P., Hu, J., Xiong, Y., Feng, D., Liu, H., Zhang, H., Yang, H., and He, J. (2011). Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) is required for the estradiol-dependent increase of phosphatase and tensin homolog (PTEN) protein expression. Endocrinology 152, 4537–4549.
Zhuang, C., Tang, H., Dissanaike, S., Cobos, E., Tao, Y., and Dai, Z. (2011). CDK1-mediated phosphorylation of Abi1 attenuates Bcr-Abl-induced F-actin assembly and tyrosine phosphorylation of WAVE complex during mitosis. J. Biol. Chem. 286, 38614–38626.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
About this article
Cite this article
Sun, C., Zheng, J., Cheng, S. et al. EBP50 phosphorylation by Cdc2/Cyclin B kinase affects actin cytoskeleton reorganization and regulates functions of human breast cancer cell line MDA-MB-231. Mol Cells 36, 47–54 (2013). https://doi.org/10.1007/s10059-013-0014-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-013-0014-0