Abstract:
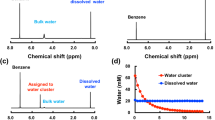
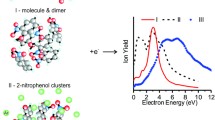
clusters (solvents being , or ) have been studied by resonance enhanced two photons ionization, leading to the detection of clusters. When water is the solvent, large clusters up to n>50 can be observed, whereas for and no clusters larger than 10 could be evidenced. Because the first step in the ionization process is the excitation from the ground solvated () ion pair state to a covalent excited state, the differences in the cluster size distribution for different solvent may be interpreted as a difference in cluster structures leading to a difference in the charge separation in the ground state.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 30 September 1997 / Revised in final form: 30 October 1997 / Accepted: 30 October 1997
Rights and permissions
About this article
Cite this article
Grégoire, G., Mons, M., Dedonder-Lardeux, C. et al. Is NaI soluble in water clusters?. Eur. Phys. J. D 1, 5–7 (1998). https://doi.org/10.1007/s100530050057
Issue Date:
DOI: https://doi.org/10.1007/s100530050057