Abstract
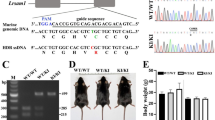
Mast syndrome (SPG21) is a childhood-onset, autosomal recessive, complicated form of hereditary spastic paraplegia (HSP) characterized by dementia, thin corpus callosum, white matter abnormalities, and cerebellar and extrapyramidal signs in addition to spastic paraparesis. A nucleotide insertion resulting in premature truncation of the SPG21 gene product maspardin underlies this disorder, likely leading to loss of protein function. In this study, we generated SPG21−/− knockout mice by homologous recombination as a possible animal model for SPG21. Though SPG21−/− mice appeared normal at birth, within several months they developed gradually progressive hind limb dysfunction. Cerebral cortical neurons cultured from SPG21−/− mice exhibited significantly more axonal branching than neurons from wild-type animals, while comprehensive neuropathological analysis of SPG21−/− mice did not reveal definitive abnormalities. Since alterations in axon branching have been seen in neurons derived from animal models of other forms of HSP as well as motor neuron diseases, this may represent a common cellular pathogenic theme.







Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- DTA:
-
Diphtheria toxin subunit A
- ES:
-
Embryonic stem
- HSP:
-
Hereditary spastic paraplegia
- MBP:
-
Myelin basic protein
- NF:
-
Neurofilament
- NGS:
-
Normal goat serum
- NMJ:
-
Neuromuscular junction
- PBS:
-
Phosphate-buffered saline
- PFA:
-
Paraformaldehyde
- TBS:
-
Tris-buffered saline
References
Harding AE (1983) Classification of the hereditary ataxias and paraplegias. Lancet 1:1151–1155
Salinas S, Proukakis C, Crosby A, Warner TT (2008) Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol 7:1127–1138
Reid E (2003) Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. J Med Genet 40:81–86
Fink JK (2006) Hereditary spastic paraplegia. Curr Neurol Neurosci Rep 6:65–76
Soderblom C, Blackstone C (2006) Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol Ther 109:42–56
Züchner S (2007) The genetics of hereditary spastic paraplegia and implications for drug therapy. Expert Opin Pharmacother 8:1433–1439
Stevanin G, Ruberg M, Brice A (2008) Recent advances in the genetics of spastic paraplegias. Curr Neurol Neurosci Rep 8:198–210
Hu J, Shibata Y, Zhu P-P, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C (2009) A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 138:549–561
Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A (2009) Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460:978–983
Park SH, Zhu P-P, Parker RL, Blackstone C (2010) Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J Clin Invest 120:1097–1110
Cross HE, McKusick VA (1967) The Mast syndrome: a recessively inherited form of presenile dementia with motor disturbances. Arch Neurol 16:1–13
Simpson MA, Cross H, Proukakis C, Pryde A, Hershberger R, Chatonnet A, Patton MA, Crosby AH (2003) Maspardin is mutated in Mast syndrome, a complicated form of hereditary spastic paraplegia associated with dementia. Am J Hum Genet 73:1147–1156
Zeitlmann L, Sirim P, Kremmer E, Kolanus W (2001) Cloning of ACP33 as a novel intracellular ligand of CD4. J Biol Chem 276:9123–9132
Hanna M, Blackstone C (2009) Interaction of the SPG21 protein ACP33/maspardin with the aldehyde dehydrogenase ALDH16A1. Neurogenetics 10:217–228
McCray BA, Skordalakes E, Taylor JP (2010) Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum Mol Genet 19:1033–1047
Zhu P-P, Patterson A, Lavoie B, Stadler J, Shoeb M, Patel R, Blackstone C (2003) Cellular localization, oligomerization, and membrane association of the hereditary spastic paraplegia 3A (SPG3A) protein atlastin. J Biol Chem 278:49063–49071
Zheng Y-L, Li B-S, Veeranna, Pant HC (2003) Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J Biol Chem 278:24026–24032
Zhu P-P, Soderblom C, Tao-Cheng J-H, Stadler J, Blackstone C (2006) SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum Mol Genet 15:1343–1353
Solowska JM, Morfini G, Falnikar A, Himes BT, Brady ST, Huang D, Baas PW (2008) Quantitative and functional analyses of spastin in the nervous system: implications for hereditary spastic paraplegia. J Neurosci 28:2147–2157
Riano E, Martignoni M, Mancuso G, Cartelli D, Crippa F, Toldo I, Siciliano G, Di Bella D, Taroni F, Bassi MT, Cappelletti G, Ruglarli EI (2009) Pleiotropic effects of spastin on neurite growth depending on expression levels. J Neurochem 108:1277–1288
Qiang L, Yu W, Liu M, Solowska JM, Baas PW (2010) Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Mol Biol Cell 21:334–344
Madduri S, Papaloïzos M, Gander B (2009) Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res 65:88–97
Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, Rubinsztein DC, Marchuk DA (2002) A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am J Hum Genet 71:1189–1194
Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R (2009) Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab 297:E289–E296
Eastman SW, Yassaee M, Bieniasz PD (2009) A role for ubiquitin ligases and spartin/SPG20 in lipid droplet turnover. J Cell Biol 184:881–894
Edwards TL, Clowes VE, Tsang HT, Connell JW, Sanderson CM, Luzio JP, Reid E (2009) Endogenous spartin (SPG20) is recruited to endosomes and lipid droplets and interacts with the ubiquitin E3 ligases AIP4 and AIP5. Biochem J 423:31–39
Windpassinger C, Auer-Grumbach M, Irobi J, Patel H, Petek E, Hörl G, Malli R, Reed JA, Dierick I, Verpoorten N, Warner TT, Proukakis C, Van den Bergh P, Verellen C, Van Maldergem L, Merlini L, De Jonghe P, Timmerman V, Crosby AH, Wagner K (2004) Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet 36:271–276
Rainier S, Bui M, Mark E, Thomas D, Tokarz D, Ming L, Delaney C, Richardson RJ, Albers JW, Matsunami N, Stevens J, Coon H, Leppert M, Fink JK (2008) Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet 82:780–785
Tsaousidou MK, Ouahchi K, Warner TT, Yang Y, Simpson MA, Laing NG, Wilkinson PA, Madrid RE, Patel H, Hentati F, Patton MA, Hentati A, Lamont PJ, Siddique T, Crosby AH (2008) Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am J Hum Genet 82:510–515
Kabashi E, Lin L, Tradewell ML, Dion PA, Bercier V, Bourgouin P, Rochefort D, Bel Hadj S, Durham HD, Vande Velde C, Rouleau GA, Drapeau P (2010) Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet 19:671–683
Acknowledgments
The authors wish to thank James Nagle and Debbie Kauffman (NINDS DNA Sequencing Facility) for DNA sequencing and Dr. Peng-Peng Zhu for generation of the phylogenetic tree. This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health [to C.S., J.S., H.J., C.B, and M.C.H.], the National Institute of General Medical Sciences Pharmacology Research Associate (PRAT) Program and the Swedish Research Council [grant numbers 13473, 20587 to O.S.].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
(GIFF 101 855 kb)
Supplementary Fig. S1
High Resolution Image (TIFF 14 936 kb)
Supplementary Fig. S2
(GIFF 120 196 kb)
Supplementary Fig. S2
High Resolution Image (TIFF 7 089 kb)
SPG21+/+ mouse during the narrow beam-walking test (WMV 1,818 kb)
SPG21−/− mouse during the narrow beam-walking test (WMV 4,115 kb)
Rights and permissions
About this article
Cite this article
Soderblom, C., Stadler, J., Jupille, H. et al. Targeted disruption of the Mast syndrome gene SPG21 in mice impairs hind limb function and alters axon branching in cultured cortical neurons. Neurogenetics 11, 369–378 (2010). https://doi.org/10.1007/s10048-010-0252-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-010-0252-7