Abstract
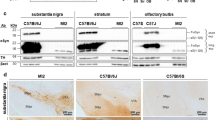
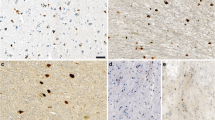
Synphilin-1 has been identified as an interacting protein of alpha-synuclein, Parkin, and LRRK2, proteins which are mutated in familial forms of Parkinson’s disease (PD). Subsequently, synphilin-1 has also been shown to be an intrinsic component of Lewy bodies in sporadic PD. In order to elucidate the role of synphilin-1 in the pathogenesis of PD, we generated transgenic mice overexpressing wild-type and mutant (R621C) synphilin-1 driven by a mouse prion protein promoter. Transgenic expression of both wild-type and the R621C variant synphilin-1 resulted in increased dopamine levels of the nigrostriatal system in 3-month-old mice. Furthermore, we found pathological ubiquitin-positive inclusions in cerebellar sections and dark-cell degeneration of Purkinje cells. Both transgenic mouse lines showed significant reduction of motor skill learning and motor performance. These findings suggest a pathological role of overexpressed synphilin-1 in vivo and will help to further elucidate the mechanisms of protein aggregation and neuronal cell death.






Similar content being viewed by others
References
Schiesling C, Kieper N, Seidel K, Krüger R (2008) Familial Parkinson’s disease—genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol 34:255–271
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840
Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269
Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C (2000) Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha-synuclein in human and transgenic mouse brain. J Neurosci 20:6365–6373
Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM (2002) Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34:521–533
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL (2002) Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53→Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA 99:8968–8973
Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E (2002) Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res 68:568–578
Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J Neurosci 26:3942–3950
Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Hörsten S, Schmidt T, Boy J, Kuhn M, Nguyen HP, Teismann P, Schulz JB, Neumann M, Pichler BJ, Reischl G, Holzmann C, Schmitt I, Bornemann A, Kuhn W, Zimmermann F, Servadio A, Riess O (2008) Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J Neurosci 28:2471–2484
Kahle PJ (2008) alpha-Synucleinopathy models and human neuropathology: similarities and differences. Acta Neuropathol 115:87–95
Chesselet MF (2008) In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol 209:22–27
Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK (2006) Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci 26:41–50
Marx FP, Holzmann C, Strauss KM, Li L, Eberhardt O, Gerhardt E, Cookson MR, Hernandez D, Farrer MJ, Kachergus J, Engelender S, Ross CA, Berger K, Schöls L, Schulz JB, Riess O, Krüger R (2003) Identification and functional characterization of a novel R621C mutation in the synphilin-1 gene in Parkinson’s disease. Hum Mol Genet 12:1223–1231
Myhre R, Klungland H, Farrer MJ, Aasly JO (2008) Genetic association study of synphilin-1 in idiopathic Parkinson’s disease. BMC Med Genet 9:19
Engelender S, Kaminsky Z, Guo X, Sharp AH, Amaravi RK, Kleiderlein JJ, Margolis RL, Troncoso JC, Lanahan AA, Worley PF, Dawson VL, Dawson TM, Ross CA (1999) Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet 22:110–114
Zaarur N, Meriin AB, Gabai VL, Sherman MY (2008) Triggering aggresome formation. Dissecting aggresome-targeting and aggregation signals in synphilin 1. J Biol Chem 283:27575–27584
Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H (2000) Synphilin-1 is present in Lewy bodies in Parkinson’s disease. Ann Neurol 47:521–523
Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM (2001) Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7:1144–1150
Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9:1231–1233
Jin HG, Yamashita H, Nakamura T, Fukuba H, Takahashi T, Hiji M, Kohriyama T, Matsumoto M (2008) Synphilin-1 transgenic mice exhibit mild motor impairments. Neurosci Lett 445:12–17
Bichelmeier U, Schmidt T, Hübener J, Boy J, Rüttiger L, Häbig K, Poths S, Bonin M, Knipper M, Schmidt WJ, Wilbertz J, Wolburg H, Laccone F, Riess O (2007) Nuclear localization of ataxin-3 is required for the manifestation of symptoms in SCA3: in vivo evidence. J Neurosci 27:7418–7428
Zhou A, Paranjape JM, Hassel BA, Nie H, Shah S, Galinski B, Silverman RH (1998) mpact of RNase L overexpression on viral and cellular growth and death. J Interferon Cytokine Res 18:953–961
Braak H, Braak E, Ohm T, Bohl J (1988) Silver impregnation of Alzheimer’s neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol 63:197–200
Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG (2003) Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem 278:44405–44411
Murray IJ, Medford MA, Guan HP, Rueter SM, Trojanowski JQ, Lee VM (2003) Synphilin in normal human brains and in synucleinopathies: studies with new antibodies. Acta Neuropathol 105:177–184
Eyal A, Szargel R, Avraham E, Liani E, Haskin J, Rott R, Engelender S (2006) Synphilin-1A: an aggregation-prone isoform of synphilin-1 that causes neuronal death and is present in aggregates from alpha-synucleinopathy patients. Proc Natl Acad Sci USA 103:5917–5922
Buitrago MM, Schulz JB, Dichgans J, Luft AR (2004) Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem 81:211–216
van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G (2000) Neuropathology in mice expressing human alpha-synuclein. J Neurosci 20:6021–6029
Freichel C, Neumann M, Ballard T, Müller V, Woolley M, Ozmen L, Borroni E, Kretzschmar HA, Haass C, Spooren W, Kahle PJ (2007) Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging 28:1421–1435
Ribeiro CS, Carneiro K, Ross CA, Menezes JR, Engelender S (2002) Synphilin-1 is developmentally localized to synaptic terminals, and its association with synaptic vesicles is modulated by alpha-synuclein. J Biol Chem 277:34651–34654
Neumann M, Muller V, Gorner K, Kretzschmar HA, Haass C, Kahle PJ (2004) Pathological properties of the Parkinson’s disease-associated protein DJ-1 in alpha-synucleinopathies and tauopathies: relevance for multiple system atrophy and Pick’s disease. Acta Neuropathol (Berl) 107:489–496
Gispert S, Del Turco D, Garrett L, Chen A, Bernard DJ, Hamm-Clement J, Korf HW, Deller T, Braak H, Auburger G, Nussbaum RL (2003) Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol Cell Neurosci 24:419–429
Krenz A, Falkenburger BH, Gerhardt E, Drinkut A, Schulz JB (2009) Aggregate formation and toxicity by wild-type and R621C synphilin-1 in the nigrostriatal system of mice using adenoviral vectors. J Neurochem 108:139–146
Turmaine M, Raza A, Mahal A, Mangiarini L, Bates GP, Davies SW (2000) Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc Natl Acad Sci USA 97:8093–8097
Petrasch-Parwez E, Nguyen HP, Löbbecke-Schumacher M, Habbes HW, Wieczorek S, Riess O, Andres KH, Dermietzel R, von Hörsten S (2007) Cellular and subcellular localization of Huntingtin aggregates in the brain of a rat transgenic for Huntington disease. J Comp Neurol 501:716–730
Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, La Spada AR (2006) Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci 9:1302–1311
Lalonde R, Strazielle C (2006) Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res 1140:51–74
Hikosaka O, Nakamura K, Sakai K, Nakahara H (2002) Central mechanisms of motor skill learning. Curr Opin Neurobiol 12:217–222
Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R (2008) Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci 27:947–957
Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ (1999) Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson’s disease A 3D [(18)F]dopa-PET study. Brain 122:1637–1650
Lotharius J, Brundin P (2002) Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum Mol Genet 11:2395–2407
Paxinos G, Franklin KB (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic, San Diego
Acknowledgement
We thank our colleagues for the fruitful discussion; in particular, Simone Engelender, Philipp Kahle, and Elisabeth Petrasch-Parwez. We would like to thank Jana Boy and Thorsten Schmidt for the kind assistance with the qPCR. The technical help with electron microscopy by Gabi Frommer-Kästle was greatly appreciated. This study was supported by a National Genome Research Network (NGFN) research grant (01GS0468) to O. Riess.
Author information
Authors and Affiliations
Corresponding author
Additional information
Silke Nuber and Thomas Franck contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Altered glucose metabolism in synphilin-1 transgenic mice. a [18F]FDG uptake measured as standard uptake volume (SUV) of various brain areas and of muscle tissue of the right and left forepaw. The graphs display from left to right the bulbus olfactorius, cerebellum, striatum, and the muscle of transgenic (WT and R621C) synphilin-1 mice compared to control mice, displaying upregulated global glucose metabolism. There is no significant difference between transgenic and control mice in the SUV of muscle tissue. b [18F]FDG uptake expressed as ratio of SUV of various brain areas to SUV of muscle tissue of the right and left forepaw. There was no significance detected in [18F]FDG uptake ratios between the tested groups. The graphs display from left to right the bulbus olfactorius/muscle, cerebellum/muscle, and striatum/muscle ratio. Error bars represent the standard deviation of each group and black stars indicate significant difference (t test; p < 0.05). (GIF 86 kb)
Rights and permissions
About this article
Cite this article
Nuber, S., Franck, T., Wolburg, H. et al. Transgenic overexpression of the alpha-synuclein interacting protein synphilin-1 leads to behavioral and neuropathological alterations in mice. Neurogenetics 11, 107–120 (2010). https://doi.org/10.1007/s10048-009-0212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-009-0212-2