Abstract
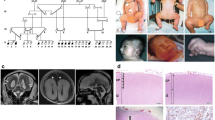
Lissencephaly spectrum (LIS) is one of the most severe neuronal migration disorders that ranges from agyria/pachygyria to subcortical band heterotopia. Approximately 80% of patients with the LIS spectrum carry mutations in either the LIS1 or DCX (doublecortin) genes which have an opposite gradient of severity. The aim of the study was to evaluate in detail the phenotype of DCX-associated lissencephaly and to look for genotype–phenotype correlations. Of the 180 male patients with DCX-related lissencephaly, 33 males (24 familial cases and nine cases with de novo mutations) were found with hemizygous DCX mutations and were clinically and genetically assessed here. DCX mutation analysis revealed that the majority of mutations were missense (79.2%), clustered in the two evolutionary conserved domains, N-DC and C-DC, of DCX. The most prominent radiological phenotype was an anteriorly predominant pachygyria or agyria (54.5%) although DCX-associated lissencephaly encompasses a complete range of LIS grades. The severity of neurological impairment was in accordance with the degree of agyria with severe cognitive impairment in all patients, inability to walk independently in over half and refractory epilepsy in more than a third. For genotype–phenotype correlations, patients were divided in two groups according to the location of DCX missense mutations. Patients with mutations in the C-DC domain tended to have a less severe lissencephaly (grade 4–5 in 58.3%) compared with those in the N-DC domain (grade 4–5 in 36.3%) although, in this dataset, this was not statistically significant (p = 0.12). Our evaluation suggests a putative correlation between phenotype and genotype. These data provide further clues to deepen our understanding of the function of the DCX protein and may give new insights into the molecular mechanisms that could influence the consequence of the mutation in the N-DC versus the C-DC domain of DCX.


Similar content being viewed by others
References
Kerjan G, Gleeson JG (2007) Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet 23:623–630. doi:10.1016/j.tig.2007.09.003
Guerrini R, Dobyns WB, Barkovich AJ (2008) Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci 31:154–162
Barkovich AJ, Koch TK, Carrol CL (1991) The spectrum of lissencephaly: report of ten patients analyzed by magnetic resonance imaging. Ann Neurol 30:139–146. doi:10.1002/ana.410300204
Reiner O, Carrozzo R, Shen Y et al (1993) Isolation of a Miller–Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature 364:717–721. doi:10.1038/364717a0
des Portes V, Pinard JM, Billuart P et al (1998) A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92:51–61. doi:10.1016/S0092-8674(00)80898-3
Gleeson JG, Allen KM, Fox JW et al (1998) Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92:63–72. doi:10.1016/S0092-8674(00)80899-5
Pilz DT, Matsumoto N, Minnerath S et al (1998) LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet 7:2029–2037. doi:10.1093/hmg/7.13.2029
Gleeson JG, Minnerath SR, Fox JW et al (1999) Characterization of mutations in the gene doublecortin in patients with double cortex syndrome. Ann Neurol 45:146–153. doi:10.1002/1531-8249(199902)45:2<146::AID-ANA3>3.0.CO;2-N
des Portes V, Francis F, Pinard JM et al (1998) Doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH). Hum Mol Genet 7:1063–1070. doi:10.1093/hmg/7.7.1063
Gleeson JG, Minnerath S, Kuzniecky RI et al (2000) Somatic and germline mosaic mutations in the doublecortin gene are associated with variable phenotypes. Am J Hum Genet 67:574–581. doi:10.1086/303043
Dobyns WB, Truwit CL, Ross ME et al (1999) Differences in the gyral pattern distinguish chromosome 17-linked and X-linked lissencephaly. Neurology 53:270–277
Matsumoto N, Leventer RJ, Kuc JA et al (2001) Mutation analysis of the DCX gene and genotype/phenotype correlation in subcortical band heterotopia. Eur J Hum Genet 9:5–12. doi:10.1038/sj.ejhg.5200548
Mei D, Parrini E, Pasqualetti M et al (2007) Multiplex ligation-dependent probe amplification detects DCX gene deletions in band heterotopia. Neurology 68:446–450. doi:10.1212/01.wnl.0000252945.75668.5d
Francis F, Koulakoff A, Boucher D et al (1999) Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23:247–256. doi:10.1016/S0896-6273(00)80777-1
Gleeson JG, Lin PT, Flanagan LA et al (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257–271. doi:10.1016/S0896-6273(00)80778-3
Sapir T, Horesh D, Caspi M et al (2000) Doublecortin mutations cluster in evolutionarily conserved functional domains. Hum Mol Genet 9:703–712. doi:10.1093/hmg/9.5.703
Aigner L, Uyanik G, Couillard-Despres S et al (2003) Somatic mosaicism and variable penetrance in doublecortin-associated migration disorders. Neurology 60:329–332. doi:10.1001/archneur.60.3.329
Aigner L, Fluegel D, Dietrich J et al (2000) Isolated lissencephaly sequence and double-cortex syndrome in a German family with a novel doublecortin mutation. Neuropediatrics 31:195–198. doi:10.1055/s-2000-7452
D'Agostino MD, Bernasconi A, Das S et al (2002) Subcortical band heterotopia (SBH) in males: clinical, imaging and genetic findings in comparison with females. Brain 125:2507–2522. doi:10.1093/brain/awf248
Poolos NP, Das S, Clark GD et al (2002) Males with epilepsy, complete subcortical band heterotopia, and somatic mosaicism for DCX. Neurology 58:1559–1562
Sakamoto M, Ono J, Okada S et al (2000) Genetic alteration of the DCX gene in Japanese patients with subcortical laminar heterotopia or isolated lissencephaly sequence. J Hum Genet 45:167–170. doi:10.1007/s100380050204
Dobyns WB, Truwit CL (1995) Lissencephaly and other malformations of cortical development: 1995 update. Neuropediatrics 26:132–147
Taylor KR, Holzer AK, Bazan JF et al (2000) Patient mutations in doublecortin define a repeated tubulin-binding domain. J Biol Chem 275:34442–34450. doi:10.1074/jbc.M007078200
Dobyns WB, Elias ER, Newlin AC et al (1992) Causal heterogeneity in isolated lissencephaly. Neurology 42:1375–1388
Moores CA, Perderiset M, Kappeler C et al (2006) Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO J 25:4448–4457. doi:10.1038/sj.emboj.7601335
Reiner O, Coquelle FM, Peter B et al (2006) The evolving doublecortin (DCX) superfamily. BMC Genomics 7:188. doi:10.1186/1471-2164-7-188
Kappeler C, Saillour Y, Baudoin JP et al (2006) Branching and nucleokinesis defects in migrating interneurons derived from doublecortin knockout mice. Hum Mol Genet 15:1387–1400. doi:10.1093/hmg/ddl062
Koizumi H, Tanaka T, Gleeson JG (2006) Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron 49:55–66. doi:10.1016/j.neuron.2005.10.040
Moores CA, Perderiset M, Francis F et al (2004) Mechanism of microtubule stabilization by doublecortin. Mol Cell 14:833–839. doi:10.1016/j.molcel.2004.06.009
Kim MH, Cierpicki T, Derewenda U et al (2003) The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat Struct Biol 10:324–333. doi:10.1038/nsb918
Moores CA, Francis F, Perderiset M et al (2003) A double-take on MAPs. Nat Struct Biol 10:314–316. doi:10.1038/nsb0503-314
Horesh D, Sapir T, Francis F et al (1999) Doublecortin, a stabilizer of microtubules. Hum Mol Genet 8:1599–1610. doi:10.1093/hmg/8.9.1599
Acknowledgements
The authors wish to thank all the families and clinicians Valérie Lieko, Marie Ange N’Guyen Morel, Isabelle Caubel, Pierre Angelo Vegiotti, Laurence Faivre Olivier, Bertrand Sotos, Anna Kaminska, Isabelle Desguerre and Sylvie Odent whose cooperation made this study possible. We thank David Keays and Drs M. Eisermann, Dr Soufflet and P Plouin for their helpful discussions and their help in the EEG analysis. This work was supported by the Société d'Etudes et de Soins pour les Enfants Paralysés et Polymalformés (CESEP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leger, PL., Souville, I., Boddaert, N. et al. The location of DCX mutations predicts malformation severity in X-linked lissencephaly. Neurogenetics 9, 277–285 (2008). https://doi.org/10.1007/s10048-008-0141-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-008-0141-5