Abstract
Muscle–eye–brain disease (MEB, OMIM 253280) is an autosomal recessive disorder characterized by a distinct triad of congenital muscular dystrophy, structural eye abnormalities, and cobblestone lissencephaly. Clinically, MEB patients present with early onset muscular hypotonia, severely compromised motor development, and mental retardation. Magnetic resonance imaging reveals a lissencephaly type II with hypoplasia of the brainstem and cerebellum. MEB is associated with mutations in the gene for protein O-mannose beta-1,2-N-acetylglucosaminyltransferase (POMGnT1, OMIM 606822). In this paper, we report the clinical findings of nine MEB patients from eight families. Eight of the nine patients presented typical features of MEB. However, a broad phenotypic variability was observed, ranging from two patients with severe autistic features to another patient with an unusually mild phenotype, initially diagnosed as congenital muscular dystrophy. Furthermore, severe hydrocephalus was reported in two families during a previous pregnancy, emphasizing the phenotypic overlap with Walker–Warburg syndrome. In addition to three previously reported mutations, we identified six novel POMGnT1 mutations (one missense, five truncating) in the present patient cohort. Our data suggest mutational hotspots within the minimal catalytic domain at arginine residue 442 (exon 16) and in intron 17. It is interesting to note that all mutations analyzed so far result in a complete loss of enzyme activity. Therefore, we conclude that the type and position of the POMGnT1 mutations are not of predictive value for the clinical severity. This supports the notion that additional environmental and/or genetic factors may contribute to the observed broad spectrum of POMGnT1-associated phenotypes.



Similar content being viewed by others
References
Lehle L, Strahl S, Tanner W (2006) Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl 45:6802–6818
Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T (2001) Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell 1:717–724
Taniguchi K, Kobayashi K, Saito K, Yamanouchi H, Ohnuma A, Hayashi YK, Manya H, Jin DK, Lee M, Parano E, Falsaperla R, Pavone P, Van Coster R, Talim B, Steinbrecher A, Straub V, Nishino I, Topaloglu H, Voit T, Endo T, Toda T (2003) Worldwide distribution and broader clinical spectrum of muscle–eye–brain disease. Hum Mol Genet 12:527–534
Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van Bokhoven H, Brunner HG (2002) Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet 71:1033–1043
van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L (2005) POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker Warburg syndrome. J Med Genet 42:907–912
Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, Hamano K, Sakakihara Y, Nonaka I, Nakagome Y, Kanazawa I, Nakamura Y, Tokunaga K, Toda T (1998) An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394:388–392
Silan F, Yoshioka M, Kobayashi K, Simsek E, Tunc M, Alper M, Cam M, Guven A, Fukuda Y, Kinoshita M, Kocabay K, Toda T (2003) A New mutation of the fukutin gene in a non-Japanese patient. Ann Neurol 53:392–396
Diesen C, Saarinen A, Pihko H, Rosenlew C, Cormand B, Dobyns WB, Dieguez J, Valanne L, Joensuu T, Lehesjoki AE (2004) POMGnT1 mutation and phenotypic spectrum in muscle–eye–brain disease. J Med Genet 41:e115
Kano H, Kobayashi K, Herrmann R, Tachikawa M, Manya H, Nishino I, Nonaka I, Straub V, Talim B, Voit T, Topaloglu H, Endo T, Yoshikawa H, Toda T (2002) Deficiency of adystroglycan in muscle–eye–brain disease. Biochem Biophys Res Commun 291:1283–1286
Santavuori P, Valanne L, Autti T, Haltia M, Pihko H, Sainio K (1998) Muscle–eye–brain disease—clinical features, visual evoked potentials and brain imaging in 20 patients. Eur J Peadiatr Neurol 1:41–47
Pihko H, Lappi M, Raitta C, Sainio K, Valanne L, Somer H, Santavuori P (1995) Ocular findings in muscle–eye–brain (MEB) disease: a follow up study. Brain Dev 17:57–61
Valanne L, Pihko H, Katevuo K, Karttunen P, Somer H, Santavuori P (1994) MRI of the brain in muscle–eye–brain (MEB) disease. Neuroradiology 36:473–476
Haliloglu G, Gross C, Senbil N, Talim B, Hehr U, Uyanik G, Winkler J, Topaloglu H (2004) Clinical spectrum of muscle–eye–brain disease: from the typical presentation to severe autistic features. Acta Myol 23:137–139
Meyer S, Struffert T, Uyanik G, Oehl-Jaschkowitz B, Hehr U, Shamdeen MG (2005) Congenital muscular dystrophies: muscle–eye–brain disease. Klin Padiatr 217:68–69 (in German)
Kanoff RJ, Curless RG, Petito C, Falcone S, Siatkowski RM, Pegoraro E (2002) Walker–Warburg syndrome: neurologic features and muscle membrane structure. Pediatr Neurol 18:76–80
Vervoort VS, Holden KR, Ukadike KC, Collins JS, Saul RA, Srivastava AK (2004) POMGnT1 gene alterations in a family with neurological abnormalities. Ann Neurol 56:143–148
Zhang W, Vajsar J, Cao P, Breningstall G, Diesen C, Dobyns W, Herrmann R, Lehesjoki A-E, Steinbrecher A, Talim B, Toda T, Topaloglu H, Voit T, Schachter H (2003) Enzymatic diagnostic test for Muscle–Eye–Brain type congenital muscular dystrophy using commercially available reagents. Clin Biochem 36:339–344
Akasaka-Manya K, Manya H, Kobayashi K, Toda T, Endoa T (2004) Structure–function analysis of human protein O-linked mannose b1,2-N-acetylglucosaminyltransferase 1, POMGnT1. Biochem Biophys Res Commun 320:39–44
Godfrey C, Mein R, Brockington M, Elson E, Topaloglu H, Smith J, Escolar D, Bertini E, Merlini I, Mercuri E, Bushby K, Straub V, North K, Abbs S, Muntoni F (2006) Molecular genetic analysis of 6 glycosyltransferases in a large population of dystroglycanopathy patients significantly widens the spectrum of phenotypes resulting from POMT1, POMGnT1 and Fukutin mutations. Neuromuscul Disord 16:683
Balci B, Uyanik G, Dincer P, Gross C, Willer T, Talim B, Haliloglu G, Kale G, Hehr U, Winkler J, Topaloglu H (2005) An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord 15:271–275
van Reeuwijk J, Maugenre S, van den Elzen C, Verrips A, Bertini E, Muntoni F, Merlini L, Scheffer H, Brunner HG, Guicheney P, van Bokhoven H (2006) The expanding phenotype of POMT1 mutations: from Walker–Warburg syndrome to congenital muscular dystrophy, microcephaly, and mental retardation. Human Mutat 27:453–459
Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T (1997) Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem 272:2156–2162
Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K (2001) Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57:115–121
Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, Imamura M, Nakamura Y, Shimizu T, Murakami T, Sunada Y, Fujikado T, Matsumura K, Terashima T, Toda T (2003) Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet 12:1449–1459
Willer T, Prados B, Falcon-Perez JM, Renner-Muller I, Przemeck GK, Lommel M, Coloma A, Valero MC, de Angelis MH, Tanner W, Wolf E, Strahl S, Cruces J (2004) Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci USA 101:14126–1431
Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H (2006) A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose 1,2-N-acetylglucosaminyltransferase (POMGnT1). Mech Dev 123:228–240
Stoltenburg-Didinger G, Steinbrecher A (2003) Morphogenesis of type II lissencephaly: neuropathology, genetics and pathomechanisms. Neuroembryology 2:32–39
Manya H, Sakai K, Kobayashi K, Taniguchi K, Kawakita M, Toda T, Endo T (2003) Loss-of-function of an N-acetylglucosaminyltransferase, POMGnT1, in muscle–eye–brain disease. Biochem Biophys Res Commun 306:93–97
Biancheri R, Bertini E, Falace A, Pedemonte M, Rossi A, D’Amico A, Scapolan S, Bergamino L, Petrini S, Cassandrini D, Broda P, Manfredi M, Zara F, Santorelli FM, Minetti C, Bruno C (2006) POMGnT1 mutations in congenital muscular dystrophy: genotype–phenotype correlation and expanded clinical spectrum. Arch Neurol 63:1491–1495
Matsumoto H, Hayashi YK, Kim DS, Ogawa M, Murakami T, Noguchi S, Nonaka I, Nakazawa T, Matsuo T, Futagami S, Campbell KP, Nishino I (2005) Congenital muscular dystrophy with glycosylation defects of alpha-dystroglycan in Japan. Neuromuscul Disord 15:342–348
Vajsar J, Zhang W, Dobyns WB, Biggar D, Holden KR, Hawkins C, Ray P, Olney AH, Burson CM, Srivastava AK, Schachter H (2006) Carriers and patients with muscle-eye-brain disease can be rapidly diagnosed by enzymatic analysis of fibroblasts and lymphoblasts. Neuromuscul Disord 16:132–136
Balci B, Morris-Rosendahl DJ, Celebi A, Talim B, Topaloglu H, Dincer P (2007) Prenatal diagnosis of muscle–eye–brain disease. Prenat Diagn 27:51–54
Acknowledgments
We thank the families for their participation in this study, B.H.F. Weber for his support and scientific advice, and C. Mai and F. Koehler for excellent technical assistance. This work was supported in part by the “Regensburger Forschungsförderung der Medizinischen Fakultät” (ReForM; University of Regensburg, Germany), the Joint German-Israeli Research Program of the Federal Ministry of Education and Research (BMBF) and the Ministry of Science and Technology (MOST) “Towards neural stem cell based therapies: the impact of neuronal migration” (BMBF no. 01GA0510). MCW and HL are members of the German Muscular Dystrophy Network (MD-NET; 01GM0601; www.md-net.org) funded by the German Ministry of Education and Research (BMBF, Bonn, Germany). MD-NET is a partner of TREAT-NMD (EC, 6th FP, proposal no. 036825; www.treat-nmd.eu).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Summary of novel POMGnT1 mutations.
ESM
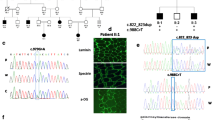
POMGnT1 missense mutations at codon 442: wild-type sequence (a), patient 2a homozygous Arg442Cys (b), and patient 7 homozygous Arg442His (c). MspI restriction fragment length polymorphism and sequence demonstrating the presence of the POMGnT1 mutation c.1540−2A>G in homozygous state in patient 5 and in heterozygous state in both parents but not in the control (d). Patient 6, homozygous for the frameshift mutation c.1350_1354delCTGGG in exon 16 of the POMGnT1 gene; (wild-type in upper lanes, e) (PDF 269 kb)
Rights and permissions
About this article
Cite this article
Hehr, U., Uyanik, G., Gross, C. et al. Novel POMGnT1 mutations define broader phenotypic spectrum of muscle–eye–brain disease. Neurogenetics 8, 279–288 (2007). https://doi.org/10.1007/s10048-007-0096-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-007-0096-y