Abstract
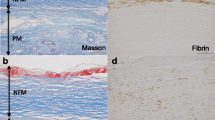
Characteristics of pathological alterations in long-term peritoneal dialysis (PD) are thickening of submesothelial compact (SMC) zone, small-vessel vasculopathy, and loss of mesothelial cells. Bioincompatible PD fluid plays crucial roles in peritoneal injury. Encapsulating peritoneal sclerosis (EPS), a rare and serious complication, occurred in patients on long-term PD or frequent peritonitis episodes, and ~50 % of EPS developed after PD cessation. We hypothesized that PD-related peritoneal injury factors induced by bioincompatible PD fluid accumulated in the peritoneum and might induce EPS. We therefore examined the accumulation of advanced glycation end products (AGE) and beta 2-microglobulin (β2M) in peritoneum and evaluated the relationship between their accumulation, clinical parameters, and outcome after PD cessation. Forty-five parietal peritoneal specimens were obtained from 28 PD patients, 14 uremic patients, and three patients with normal kidney function. The peritoneal equilibration test was used for peritoneal function. AGE- and β2M-expressing areas were found in vascular walls, perivascular areas, and the deep layer of the SMC in short-term PD patients and extended over the entire SMC in long-term patients. Peritonitis and prolonged PD treatment aggravated peritoneal thickening and the proportion of AGE-expressing areas. The proportion of β2M-expressing areas was increased in long-term PD patients. Thickening of the SMC and the proportions of AGE- and β2M-expressing areas were not related to ascites or EPS after PD withdrawal. It appears that the increased proportion of AGE and β2M deposition induced by long-term exposure of PD fluid may be a marker of peritoneal injury.



Similar content being viewed by others
References
Di Paolo N, Sacchi G, De Mia M, et al. Morphology of the peritoneal membrane during continuous ambulatory peritoneal dialysis. Nephron. 1986;44:204–11.
Gotloib L, Shostak A. Ultrastructural morphology of the peritoneum: new findings and speculations on transfer of solutes and water during peritoneal dialysis. Perit Dial Bull. 1987;7:119–29.
Honda K, Nitta K, Horita S, Yumura W, Nihei H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron. 1996;72:171–6.
Williams JD, Craig KJ, Topley N, et al. Peritoneal Biopsy Study Group: morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–9.
Kawanishi H, Kawaguchi Y, Fukui H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis. 2004;44:729–37.
Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25:S19–29.
Nakayama M, Kawaguchi Y, Yamada K, et al. Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int. 1997;51:182–6.
Ohashi K. Pathogenesis of beta2-microglobulin amyloidosis. Pathol Int. 2001;51:1–10.
Ohashi K, Hara M, Kawai R, et al. Cervical discs are most susceptible to beta2-microglobulin amyloid deposition in the vertebral column. Kidney Int. 1992;41:1646–52.
Argiles A, Mourad G, Kerr PG, et al. Cells surrounding haemodialysis-associated amyloid deposits are mainly macrophages. Nephrol Dial Transplant. 1994;9:662–7.
Yokoyama K, Yoshida H, Matsuo N, et al. Serum beta2 microglobulin (beta2MG) level is a potential predictor for encapsulating peritoneal sclerosis (EPS) in peritoneal dialysis patients. Clin Nephrol. 2008;69:121–6.
Eichner T, Radford SE. Understanding the complex mechanisms of β2-microglobulin amyloid assembly. FEBS J. 2011;278:3868–83.
Osada S, Hamada C, Shimaoka T, et al. Alterations in proteoglycan components and histopathology of the peritoneum in uraemic and peritoneal dialysis (PD) patients. Nephrol Dial Transplant. 2009;24:3504–12.
Yamaguchi I, Suda H, Tsuzuike N, et al. Glycosaminoglycan and proteoglycan inhibit the depolymerization of beta2-microglobulin amyloid fibrils in vitro. Kidney Int. 2003;64:1080–8.
Lo WK, Kawanishi H. Encapsulating peritoneal sclerosis–medical and surgical treatment. Perit Dial Int. 2009;29 Suppl 2:S211–4.
Twardowski ZJ, Nolph kDa, Khanna R, et al. Peritoneal equilibration test. Perit Dial Bull. 1987;7:138–47.
Ikeda K, Higashi T, Sano H, et al. N(epsilon)-(carboxymethyl) lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996;35:8075–83.
Solé M, Muñoz-Gómez J, Campistol JM. Role of amyloid in dialysis-related arthropathies. A morphological analysis of 23 cases. Virchows Arch A Pathol Anat Histopathol. 1990;417:523–8.
Shimaoka T, Hamada C, Kaneko K, et al. Quantitative evaluation and assessment of peritoneal morphologic changes in peritoneal dialysis patients. Nephrol Dial Transplant. 2010;10:3379–85.
Nakamura S, Miyazaki S, Sasaki S, et al. Localization of imidazolone in the peritoneum of CAPD patients: a factor for a loss of ultrafiltration. Am J Kidney Dis. 2001;38:S107–10.
Miyata T, Horie K, Ueda Y, et al. Advanced glycation and lipid oxidation of the peritoneal membrane: respective roles of serum and peritoneal fluid reactive carbonyl compounds. Kidney Int. 2000;58:425–35.
Honda K, Hamada C, Nakayama M, Peritoneal Biopsy Study Group of the Japanese Society for Peritoneal Dialysis, et al. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol. 2008;3:720–8.
Park MS, Lee HA, Chu WS, et al. Peritoneal accumulation of AGE and peritoneal membrane permeability. Perit Dial Int. 2000;20:452–60.
Brownlee M, Vlassara H, Cerami M. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–37.
Bucciarelli LG, Ananthakrishnan R, Hwang YC, et al. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–51.
De Vriese AS, Tilton RG, Mortier S, Lameire NH. Myofibroblast transdifferentiation of mesothelial cells is mediated by RAGE and contributes to peritoneal fibrosis in uraemia. Nephrol Dial Transplant. 2006;21:2549–55.
Lai KN, Lai KB, Lam CW, et al. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;35:644–52.
Naiki H, Yamamoto S, Hasegawa K, et al. Molecular interactions in the formation and deposition of beta2-microglobulin-related amyloid fibrils. Amyloid. 2005;12:15–25.
Miyata T, Inagi R, Iida Y, et al. Involvement of beta 2-microglobulin modified with advanced glycation end products in the pathogenesis of hemodialysis-associated amyloidosis. Induction of human monocyte chemotaxis and macrophage secretion of tumor necrosis factor-alpha and interleukin-1. J Clin Invest. 1994;93:521–8.
Conflict of interest
We have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Deceased: H. Nakamoto.
Rights and permissions
About this article
Cite this article
Nakamoto, H., Hamada, C., Shimaoka, T. et al. Accumulation of advanced glycation end products and beta 2-microglobulin in fibrotic thickening of the peritoneum in long-term peritoneal dialysis patients. J Artif Organs 17, 60–68 (2014). https://doi.org/10.1007/s10047-013-0741-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-013-0741-1