Abstract
The frequency of autosomal-dominant cerebellar ataxia (ADCA) subtypes was examined in 86 unrelated families originating from Nagano prefecture. In Nagano, the prevalence of spinocerebellar degeneration (SCD) was approximately 22 per 100,000 population. Among ADCA families, SCA6 was the most prevalent subtype (16 families, 19%), followed by DRPLA (nine families, 10%), SCA3/MJD (three families, 3%), SCA1 (two families, 2%), and SCA2 (one family, 1%). No families with SCA7, SCA12, or SCA17 were detected. Compared with other districts in Japan, the prevalence of SCA3/MJD was very low in Nagano. More interestingly, the ratio of genetically undetermined ADCA families was much higher in Nagano (55 families, 65%) than in other districts in Japan. These families tended to accumulate in geographically restricted areas such as Kiso, Saku, and Ina, indicating that the founder effect might be responsible for the high frequency of ADCA in these areas. Most patients clinically showed slowly progressive pure cerebellar ataxia of late-onset (ADCA III). In the case of 36 patients from 36 genetically undetermined ADCA III families, however, no one was completely consistent with the founder allele proposed for 16q-ADCA. These results indicate that there might be genetically distinct ADCA subtypes in Nagano.
Similar content being viewed by others
Introduction
The autosomal-dominant cerebellar ataxias (ADCAs) are clinically and genetically a heterogeneous group of neurodegenerative disorders characterized by variable degrees of cerebellar and brainstem dysfunction. To date, causative genes or gene loci responsible for at least 20 ADCAs have been identified. Expansions of triplet or pentanucleotide repeat have been found to be the pathogenic mutations for several forms of ADCAs, including spinocerebellar ataxia type 1 (SCA1) (Orr et al. 1993), SCA2 (Imbert et al. 1996; Pulst et al. 1996; Sanpei et al. 1996), SCA3/Machado–Joseph disease (SCA3/MJD) (Kawaguchi et al. 1994), SCA6 (Zhuchenko et al. 1997), SCA7 (David et al. 1997), SCA10 (Matsuura et al. 2000), SCA12 (Holmes et al. 1999), SCA17 (Nakamura et al. 2001), and DRPLA (Koide et al. 1994; Nagafuchi et al. 1994). More recently, point mutations in the protein kinase C gamma gene (PRKCG) have been proven to be pathogenic for SCA14 (van de Warrenburg et al. 2003; Yabe et al. 2003). Eight additional gene loci have also been mapped: SCA4 on 16q (Flanigan et al. 1996), SCA5 on 11p (Ranum et al. 1994), SCA11 on 15q (Worth et al. 1999), SCA15 on 3p (Knight et al. 2003), SCA16 on 8q (Miyoshi et al. 2001), SCA19 on 1p (Verbeek et al. 2002), SCA21 on 7p (Vuillaume et al. 2002), SCA22 on 1p (Chung et al. 2003), and SCA 25 on 2p (Stevanin et al. 2004).
In Japan, the prevalence of spinocerebellar degeneration/ataxia (SCD/SCA) including multiple-system atrophy (MSA) is 15.68 per 100,000 (Sasaki et al. 2003). About 40% of SCD patients are classified as hereditary forms, most of them showing autosomal-dominant inheritance. Several studies have indicated that SCA6, SCA3/MJD, and DRPLA are the most prevalent subtypes of ADCA in Japan despite a considerable variation in the frequency of each subtype among districts (Sasaki et al. 2003). The frequency of ADCA subtypes also varies among countries (Jardim et al. 2001; Tang et al. 2000; Stevanin et al. 1997; van de Warrenburg et al. 2002; Silveira et al. 1998; Soong et al. 2001).
Nagano prefecture, the central district of the main island of Japan, is located in a mountainous area surrounded by the Japan Alps. From geographical, historical, and cultural viewpoints, it has been divided into four different areas, the Northern (Hokushin), Eastern (Toshin), Central (Chushin), and Southern areas (Nanshin). This characteristic may be one of the factors responsible for a considerable accumulation of families with particular inherited diseases in restricted areas in Nagano. For example, there are well-known foci for transthyretin-type familial amyloidotic polyneuropathy (FAP) or autosomal-dominant amyotrophic lateral sclerosis (Ikeda et al. 1987; Fujimori et al. 1979).
To determine the frequency of ADCA subtypes in Nagano, we examined patients from 86 ADCA families and screened for mutations for SCA1, SCA2, SCA3/MJD, SCA6, SCA7, SCA12, SCA17, and DRPLA.
Materials and methods
Subjects
Clinical data from 112 patients from 86 ADCA families and 26 patients without apparent family history were collected. They were all reported to originate from Nagano prefecture. The diagnosis of ADCAs was made by experienced neurologists. Dominant inheritance was presumed when affected individuals with cerebellar ataxia were observed in at least two generations. Patients with familial spastic paraplegia or with sporadic MSA were not included in this study. Genetically undetermined families were tentatively classified according to the criteria of Harding (1993). The classification was mainly based on data of the proband when it was ambiguous because of some differences in clinical symptoms in affected members in the same family. ADCA III was defined as pure cerebellar ataxia with late onset (50 years or over) (Harding 1993), so it was excluded for such families that included at least an affected individual with onset before 40 years of age. The study was approved by the Ethics Committee of Shinshu University School of Medicine.
Molecular analysis
Informed consent was obtained prior to molecular analysis from all individuals studied. Genomic DNA was extracted from peripheral blood leukocytes with a PUREGENE DNA purification kit (Gentra). Screening for CAG repeat expansion for SCA1, SCA2, SCA3/MJD, SCA6, SCA7, SCA12, SCA17, and DRPLA was performed by PCR. The PCR reaction mixture contained the following components: 10 μl of 2× GC buffer (Takara), 200 μM each of dNTP, 10 pmol of each primer (one of the primers was labeled with Cy5), 100 ng genomic DNA and 0.25 U of LA Taq (Takara) in 20 μl. Initial denaturation at 95°C for 1 min was followed by 30 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 60°C, extension for 30 s at 72°C, and a final extension step at 72°C for 7 min. The gene-specific primers used in this study have already been published (Orr et al. 1993; Imbert et al. 1996; Pulst et al. 1996; Sanpei et al. 1996; Kawaguchi et al. 1994; Zhuchenko et al. 1997; David et al. 1997; Holmes et al. 1999; Nakamura et al. 2001; Koide et al. 1994; Nagafuchi et al. 1994). The PCR products were run with an ALF express Sizer 50–500 (Pharmacia Bioscience) in Urea gel (final conc. 7 M), including Long Ranger (FMC Bioproducts, final conc. 8%) by ALF express DNA sequencer (Pharmacia Bioscience), and the size of the products was analyzed by AlleleLinks software. In this study, the SCA8, SCA10, and SCA14 mutations were not analyzed.
According to the methods described by Takashima et al. (2001) and Li et al. (2003), we screened 36 patients from 36 genetically undetermined ADCA III families for 16q-linked ADCA (16q-ADCA). Seven microsatellite markers (TTCC01, GATA01, TTTA001, CTTT01, D16S496, D16S3067, and GT01) were used for analyzing alleles constructing a common founder haplotype for 16q-ADCA (Takashima et al. 2001; Li et al. 2003). The PCR mixture and conditions and fragment analysis were basically the same as described above, with slight modification.
Results
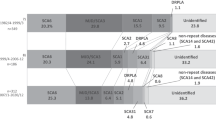
Before molecular analysis, we investigated the prevalence of SCD in Nagano prefecture. By October 2003, 491 personal check-sheets for SCD were collected in the prefecture (population 2,211,949 in 2004). This sheet is authorized by the Ministry of Health, Labour, and Welfare, and has been recently revised to exclude MSA. Thus, the prevalence of SCD not including MSA was at least 22 per 100,000 in Nagano. The prevalence was approximately ten per 100,000 in the Hokushin, 25 in the Toshin, 29 in the Chushin, and 28 in the Nanshin areas (Fig. 1). The prevalence was much higher in particularly restricted areas such as Kiso (58 per 100,000), Saku (35), and Ina (32) (Fig. 1).
The prevalence of spinocerebellar degeneration (SCD) in Nagano prefecture. The numbers indicate the number of patients with SCD per 100,000 population in four different areas (Hokushin, Toshin, Chushin, and Nanshin) in Nagano. The numbers are also specified in particular endemic areas such as Kiso, Saku, and Ina
A total of 112 patients from 86 ADCA families were examined for eight different SCA mutations, and 31 families (35%) were positive for disease-causing repeat expansions (Table 1). Twenty-four patients from 16 families (19%) were shown to have the SCA6 mutation. The DRPLA mutation was detected in 11 patients from nine families (10%), the SCA3/MJD mutation in four patients from three families (3%), the SCA1 mutation in two patients from two families (2%), and the SCA2 mutation in a patient from one family (1%). No SCA7, SCA12, or SCA17 mutations were detected. The remaining 70 patients from 55 families (65%) were genetically unidentified (Table 1). Furthermore, the ratios of genetically unknown families were much higher in the Kiso (14/15 families, 93%), Saku (13/14, 93%), and Ina areas (10/13, 77%) where the prevalence of SCD was quite high. Of the 26 patients without an apparent family history, only three (12%) were found to carry disease-causing repeat expansions: two for SCA6 and one for SCA3/MJD.
Clinical features of the 70 patients from 55 genetically undetermined ADCA families are summarized in Table 2. Of the 55 families, two (4%) and 45 (81%) were classified into ADCA type I and type III, respectively. The remaining eight families (15%) were classified into the others, and no family was found to be compatible with ADCA type II. The mean ages of onset were 43.0 in ADCA I, 60.0 in ADCA III, and 17.4 in the others. In most patients (54/70, 77%), the initial symptom was standing or gait disturbance. In 45 families with ADCA III, cerebellar ataxia of late-onset with no other particular symptoms was a predominant feature. In eight families in the others, the reasons for the classification were pure cerebellar ataxia with onset before 30 years of age (five families), higher brain dysfunction and childhood-onset cerebellar ataxia with marked genetic anticipation (one family), DRPLA-like symptoms (one family), and cerebellar ataxia plus vertical gaze impairment and mental retardation of infantile onset (one family). Only five families out of all 55 showed apparent genetic anticipation.
We screened 36 patients from 36 ADCA III families for founder alleles of 16q-ADCA. Investigation with seven different microsatellite markers showed no one was completely consistent with the founder haplotype (Table 3). The frequency of alleles shared among 16q-ADCA patients was almost the same in our patients as in general populations (Li et al. 2003), except for an allele detected by D16S3067.
Discussion
Among the genetically determined families (35%), SCA6 is the most common ADCA (19%) in Nagano, followed by DRPLA (10%), SCA3/MJD (3%), SCA1 (2%), and SCA2 (1%). Compared with the averaged frequency of ADCA subtypes in other districts of Japan (Table 1) (Takano et al. 1998; Sasaki et al. 2003), the low frequency of SCA3/MJD is characteristic of Nagano. SCA3/MJD is the most prevalent ADCA subtype in Japan (Table 1) as well as in the majority of countries (Jardim et al. 2001; Tang et al. 2000; Stevanin et al. 1997; van de Warrenburg et al. 2002; Silveira et al. 1998; Soong et al. 2001). This is also the case in the Niigata (43%) and Nagoya (39%) districts, which are geographically adjacent to Nagano; therefore, the low frequency of SCA3/MJD in Nagano (3%) is noteworthy. As reported in most districts in Japan, the frequency of SCA1 and SCA2 is also low in Nagano. Two families with SCA1 originated from the Hokushin area where families with Iiyama-type FAP (FAP accompanied by SCA1) have accumulated (Ikeda et al. 1996) although we could not confirm any relationship between the two SCA1 families described in this study and families known to have Iiyama-type FAP.
More importantly, the ratio of genetically undetermined ADCA families was much higher (65%) in Nagano. This ratio was particularly high in the Kiso, Saku, and Ina areas. The undetermined families have thus not dispersed throughout Nagano but have concentrated mainly in the localized areas described above. These areas are particularly high in the prevalence of SCD, with 58 per 100,000 in Kiso, 35 per 100,000 in Saku, and 32 per 100,000 in Ina. Geographically, population mobility has been lower in these areas, and ancestral mutations have distributed themselves inside these localized areas through the generations. The existence of these endemic areas likely explains the fact that the prevalence of SCD is much higher in Nagano than in other districts of Japan.
Forty-five (81%) of the 55 genetically undetermined families revealed pure cerebellar ataxia of late onset and were classified as ADCA III based on the criteria of Harding (1993). In the patients in this group, neurological symptoms other than cerebellar ataxia were less remarkable, but some showed hyperreflexia (23%), hyporeflexia (13%), or positive Babinski reflex (4%). Limited eye movement was observed in two patients (4%), aged 87 and 88, and might be associated with the long duration of illness (20 and 28 years). Similarly, horizontal nystagmus, observed in only 19 patients (35%), increased in frequency with duration of illness: 33% (from 0 to 10 years), 37% (from 11 to 20 years), and 67% (over 21 years). Some aged patients showed rigidity, bradykinesia, dementia, numbness of the extremities, or impaired hearing, but it was quite likely that these symptoms were caused by aging or complications such as cerebrovascular disease and degenerative spine disease.
Recently, a novel form of cerebellar ataxia linked to 16q (16q-ADCA) has been proposed (Takashima et al. 2001; Li et al. 2003; Mizusawa et al. 2004). Mizusawa et al. indicated that 16q-ADCA is the third-most prevalent subtype of ADCA next to SCA6 and SCA3/MJD in Japan and that it is distributed nationwide (Mizusawa et al. 2004). 16q-ADCA is clinically characterized by late-onset pure cerebellar ataxia; therefore, we screened genetically undetermined ADCA families in Nagano for 16q-ADCA, but no one had all the founder alleles according to seven microsatellite markers. Our results do not necessarily imply that 16q-ADCA is uncommon in Nagano because the markers used in this study are known to be distributed in the region over 3 Mb, and recombination events may occur in this region. Thus, there is a possibility that unknown ADCA families in Nagano might belong to the 16q-ADCA subtype with a haplotype different from a common founder haplotype. The identification of the 16q-ADCA gene will be needed to further clarify the gene frequency of ADCAs in Nagano.
In summary, the frequency of SCD is high in Nagano prefecture. This is probably explained by the fact that genetically undetermined ADCA families have accumulated in certain geographically restricted areas. These families are relatively similar in clinical features within particular areas. It is likely, therefore, that founder effects may have contributed to the high ratio of genetically undetermined ADCA families in each area of interest. Further, genome-wide linkage analysis is now in progress on these families.
References
Chung MY, Lu YC, Cheng NC, Soong BW (2003) A novel autosomal dominant spinocerebellar ataxia (SCA22) linked to chromosome 1p21–q23. Brain 126:1293–1299
David G, Abbas N, Stevanin G, Durr A, Yvert G, Cancel G, Weber C, Imbert G, Saudou F, Antoniou E, Drabkin H, Gemmill R, Giunti P, Benomar A, Wood N, Ruberg M, Agid Y, Mandel JL, Brice A (1997) Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet 17:65–70
Flanigan K, Gardner K, Alderson K, Galster B, Otterud B, Leppert MF, Kaplan C, Ptacek LJ (1996) Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): clinical description and genetic localization to chromosome 16q22.1. Am J Hum Genet 59:392–399
Fujimori N, Kagaya H, Yanagisawa N, Tsukagoshi H (1979) Epidemiologic investigation of motor neuron disease in the southern part of Nagano prefecture. Clin Neurol (Tokyo) 19:91–97 (In Japanese)
Harding AE (1993) Clinical features and classification of inherited ataxias. Adv Neurol 61:1–14
Holmes SE, O’Hearn EE, McInnis MG, Gorelick-Feldman DA, Kleiderlein JJ, Callahan C, Kwak NG, Ingersoll-Ashworth RG, Sherr M, Sumner AJ, Sharp AH, Ananth U, Seltzer WK, Boss MA, Vieria-Saecker AM, Epplen JT, Riess O, Ross CA, Margolis RL (1999) Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nat Genet 23:391–392
Ikeda S, Hanyu N, Hongo M, Yoshioka J, Oguchi H, Yanagisawa N, Kobayashi T, Tsukagoshi H, Ito N, Yokota T (1987) Hereditary generalized amyloidosis with polyneuropathy. Clinicopathological study of 65 Japanese patients. Brain 110:315–337
Ikeda S, Yanagisawa N, Hanyu N, Furihata K, Kobayashi T (1996) Coexistence of type I familial amyloid polyneuropathy and spinocerebellar ataxia type 1. Clinical and genetic studies of a Japanese family. J Neurol Neurosurg Psychiatry 60:586–588
Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, Weber C, Mandel JL, Cancel G, Abbas N, Durr A, Didierjean O, Stevanin G, Agid Y, Brice A (1996) Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet 14:285–291
Jardim LB, Silveira I, Pereira ML, Ferro A, Alonso I, do Ceu Moreira M, Mendonca P, Ferreirinha F, Sequeiros J, Giugliani R (2001) A survey of spinocerebellar ataxia in South Brazil—66 new cases with Machado–Joseph disease, SCA7, SCA8, or unidentified disease-causing mutations. J Neurol 248:870–876
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I et al (1994) CAG expansions in a novel gene for Machado–Joseph disease at chromosome 14q32.1. Nat Genet 8:221–228
Knight MA, Kennerson ML, Anney RJ, Matsuura T, Nicholson GA, Salimi-Tari P, Gardner RJ, Storey E, Forrest SM (2003) Spinocerebellar ataxia type 15 (sca15) maps to 3p24.2-3pter: exclusion of the ITPR1 gene, the human orthologue of an ataxic mouse mutant. Neurobiol Dis 13:147–157
Koide R, Ikeuchi T, Onodera O, Tanaka H, Igarashi S, Endo K, Takahashi H, Kondo R, Ishikawa A, Hayashi T, Saito M, Tomoda A, Miike T, Naito H, Ikuta F, Tsuji S (1994) Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet 6:9–13
Li M, Ishikawa K, Toru S, Tomimitsu H, Takashima M, Goto J, Takiyama Y, Sasaki H, Imoto I, Inazawa J, Toda T, Kanazawa I, Mizusawa H (2003) Physical map and haplotype analysis of 16q-linked autosomal dominant cerebellar ataxia (ADCA) type III in Japan. J Hum Genet 48:111–118
Matsuura T, Yamagata T, Burgess DL, Rasmussen A, Grewal RP, Watase K, Khajavi M, McCall AE, Davis CF, Zu L, Achari M, Pulst SM, Alonso E, Noebels JL, Nelson DL, Zoghbi HY, Ashizawa T (2000) Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10. Nat Genet 26:191–194
Miyoshi Y, Yamada T, Tanimura M, Taniwaki T, Arakawa K, Ohyagi Y, Furuya H, Yamamoto K, Sakai K, Sasazuki T, Kira J (2001) A novel autosomal dominant spinocerebellar ataxia (SCA16) linked to chromosome 8q22.1–24.1. Neurology 57:96–100
Mizusawa H, Ishikawa K, Toru S, Li M (2004) The prevalence and clinical features of 16q-linked autosomal dominant cerebellar ataxia (ADCA) type III in Japan. Annual report of the research committee of ataxic disease, Research on specific disease. The Ministry of Health, Labour, and Welfare of Japan, pp 67–70 (In Japanese)
Nagafuchi S, Yanagisawa H, Sato K, Shirayama T, Ohsaki E, Bundo M, Takeda T, Tadokoro K, Kondo I, Murayama N et al (1994) Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet 6:14–18
Nakamura K, Jeong SY, Uchihara T, Anno M, Nagashima K, Nagashima T, Ikeda S, Tsuji S, Kanazawa I (2001) SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet 10:1441–1448
Orr HT, Chung MY, Banfi S, Kwiatkowski TJ Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LP, Zoghbi HY (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4:221–226
Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, Pearlman S, Starkman S, Orozco-Diaz G, Lunkes A, DeJong P, Rouleau GA, Auburger G, Korenberg JR, Figueroa C, Sahba S (1996) Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 14:269–276
Ranum LP, Schut LJ, Lundgren JK, Orr HT, Livingston DM (1994) Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nat Genet 8:280–284
Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, Wakisaka A, Tashiro K, Ishida Y, Ikeuchi T, Koide R, Saito M, Sato A, Tanaka T, Hanyu S, Takiyama Y, Nishizawa M, Shimizu N, Nomura Y, Segawa M, Iwabuchi K, Eguchi I, Tanaka H, Takahashi H, Tsuji S (1996) Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet 14:277–284
Sasaki H, Yabe I, Tashiro K (2003) The hereditary spinocerebellar ataxias in Japan. Cytogenet Genome Res 100:198–205
Silveira I, Coutinho P, Maciel P, Gaspar C, Hayes S, Dias A, Guimaraes J, Loureiro L, Sequeiros J, Rouleau GA (1998) Analysis of SCA1, DRPLA, MJD, SCA2, and SCA6 CAG repeats in 48 Portuguese ataxia families. Am J Med Genet 81:134–138
Soong BW, Lu YC, Choo KB, Lee HY (2001) Frequency analysis of autosomal dominant cerebellar ataxias in Taiwanese patients and clinical and molecular characterization of spinocerebellar ataxia type 6. Arch Neurol 58:1105–1109
Stevanin G, Durr A, David G, Didierjean O, Cancel G, Rivaud S, Tourbah A, Warter JM, Agid Y, Brice A (1997) Clinical and molecular features of spinocerebellar ataxia type 6. Neurology 49:1243–1246
Stevanin G, Bouslam N, Thobois S, Azzedine H, Ravaux L, Boland A, Schalling M, Broussolle E, Durr A, Brice A (2004) Spinocerebellar ataxia with sensory neuropathy (SCA25) maps to chromosome 2p. Ann Neurol 55:97–104
Takano H, Cancel G, Ikeuchi T, Lorenzetti D, Mawad R, Stevanin G, Didierjean O, Durr A, Oyake M, Shimohata T, Sasaki R, Koide R, Igarashi S, Hayashi S, Takiyama Y, Nishizawa M, Tanaka H, Zoghbi H, Brice A, Tsuji S (1998) Close associations between prevalences of dominantly inherited spinocerebellar ataxias with CAG-repeat expansions and frequencies of large normal CAG alleles in Japanese and Caucasian populations. Am J Hum Genet 63:1060–1066
Takashima M, Ishikawa K, Nagaoka U, Shoji S, Mizusawa H (2001) A linkage disequilibrium at the candidate gene locus for 16q-linked autosomal dominant cerebellar ataxia type III in Japan. J Hum Genet 46:167–171
Tang B, Liu C, Shen L, Dai H, Pan Q, Jing L, Ouyang S, Xia J (2000) Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds. Arch Neurol 57:540–544
Verbeek DS, Schelhaas JH, Ippel EF, Beemer FA, Pearson PL, Sinke RJ (2002) Identification of a novel SCA locus (SCA19) in a Dutch autosomal dominant cerebellar ataxia family on chromosome region 1p21–q21. Hum Genet 111:388–393
Vuillaume I, Devos D, Schraen-Maschke S, Dina C, Lemainque A, Vasseur F, Bocquillon G, Devos P, Kocinski C, Marzys C, Destee A, Sablonniere B (2002) A new locus for spinocerebellar ataxia (SCA21) maps to chromosome 7p21.3–p15.1. Ann Neurol 52:666–670
van de Warrenburg BP, Sinke RJ, Verschuuren-Bemelmans CC, Scheffer H, Brunt ER, Ippel PF, Maat-Kievit JA, Dooijes D, Notermans NC, Lindhout D, Knoers NV, Kremer HP (2002) Spinocerebellar ataxias in the Netherlands: prevalence and age at onset variance analysis. Neurology 58:702–708
van de Warrenburg BP, Verbeek DS, Piersma SJ, Hennekam FA, Pearson PL, Knoers NV, Kremer HP, Sinke RJ (2003) Identification of a novel SCA14 mutation in a Dutch autosomal dominant cerebellar ataxia family. Neurology 61:1760–1765
Worth PF, Giunti P, Gardner-Thorpe C, Dixon PH, Davis MB, Wood NW (1999) Autosomal dominant cerebellar ataxia type III: linkage in a large British family to a 7.6-cM region on chromosome 15q14–21.3. Am J Hum Genet 65:420–426
Yabe I, Sasaki H, Chen DH, Raskind WH, Bird TD, Yamashita I, Tsuji S, Kikuchi S, Tashiro K (2003) Spinocerebellar ataxia type 14 caused by a mutation in protein kinase C gamma. Arch Neurol 60:1749–1751
Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC (1997) Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 15:62–69
Acknowledgements
The authors are deeply grateful to patients for their participation in this study and to Drs. Atsushi Inoue, Kosuke Naito (Kiso Prefectural Hospital), Hiroyuki Yahikozawa, Masashi Yamazaki, Shun’ichi Sato (Nagano Red Cross Hospital), Katsuhiko Kayanuma (Ina Central hospital), Ken’ichi Tabata, Jun Miki (Saku General Hospital), Keiko Maruyama (Suwa Red Cross Hospital), Teruaki Iwahashi (National Nagano Hospital), and Hiroshi Morita, Yo-ichi Takei (Shinshu University Hospital) for providing us with clinical information. We also thank Drs. Kinya Ishikawa, Shuta Toru, and Hidehiro Mizusawa (Department of Neurology, Tokyo Medical and Dental University) for their kind support of our research. This work was supported by grants from the Research Committee of Ataxic Disease (KY), Research on Specific Disease, the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimizu, Y., Yoshida, K., Okano, T. et al. Regional features of autosomal-dominant cerebellar ataxia in Nagano: clinical and molecular genetic analysis of 86 families. J Hum Genet 49, 610–616 (2004). https://doi.org/10.1007/s10038-004-0196-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-004-0196-6
Keywords
This article is cited by
-
High frequency of Machado-Joseph disease identified in Southeastern Chinese kindreds with spinocerebellar ataxia
BMC Medical Genetics (2010)
-
Analysis of an insertion mutation in a cohort of 94 patients with spinocerebellar ataxia type 31 from Nagano, Japan
neurogenetics (2010)
-
Severity and Progression Rate of Cerebellar Ataxia in 16q-linked Autosomal Dominant Cerebellar Ataxia (16q-ADCA) in the Endemic Nagano Area of Japan
The Cerebellum (2009)
-
Spectrum and prevalence of autosomal dominant spinocerebellar ataxia in Hokkaido, the northern island of Japan: a study of 113 Japanese families
Journal of Human Genetics (2007)
-
A −16C>T substitution in the 5′ UTR of the puratrophin-1 gene is prevalent in autosomal dominant cerebellar ataxia in Nagano
Journal of Human Genetics (2006)