Abstract
Moyamoya disease (MIM 252350) is characterized by stenosis or occlusion of the terminal portions of the bilateral internal carotid arteries and by abnormal vascular networks at the base of the brain. There is a high incidence of moyamoya disease in Asia, especially in Japan. Multifactorial inheritance is estimated with λs>40. Previous linkage studies have indicated that susceptibility loci for the disease are located on chromosomes 3p, 6q, and 17q. In the present study, we searched for loci linked to the disease in 12 Japanese families using 428 microsatellite markers and found significant evidence for linkage to 8q23 [maximum LOD score (MLS) of 3.6] and suggestive evidence for linkage to 12p12 (MLS=2.3). The present study revealed a novel locus for moyamoya disease.
Similar content being viewed by others
Introduction
Moyamoya disease is a specific chronic cerebrovascular occlusive disorder (Fukui et al. 2000). Stenosis of the intracranial portion of the internal carotid artery leads to secondary establishment of intracranial compensatory anastomoses at different levels (leptomeninges, basal ganglia, and transdural). The peak age of onset is 10–14 years, with a smaller peak of onset age in the 40s. The incidence of moyamoya disease is high in Japan (Ikezaki et al. 1997), but there is no region of particularly high incidence (Goto and Yonekawa 1992). The prevalence and incidence are 3.16 and 0.35 per 100,000 Japanese persons, respectively. Gender ratio (females to males) of patients is 1.8 (Ikezaki 2001). Family history of moyamoya disease is found in approximately 10% of patients (Wakai et al. 1997).
The estimated prevalence of moyamoya disease in the entire Japanese population is less than 0.01%. The prevalence of moyamoya disease in offspring and in siblings of patients with the disease has been estimated at 2.4 and 3%, respectively. Both are much higher than the prevalence in the general population (Yamauchi et al. 1997), providing a rough estimation of a λs>40 (Osawa et al. 1992). The incidence of the disease in both monozygotic twins is 80%. Multifactorial genetic inheritance is most likely (Fukui et al. 2000).
There have been several linkage studies of families with moyamoya disease. Ikeda and colleagues reported linkage of the disease to markers located on 3p26–p24.2 (Ikeda et al. 1999). Inoue and colleagues examined linkage to microsatellite markers on chromosome 6 and suggested that marker D6S441 on 6q25 is linked to the disease (Inoue et al. 2000). Yamauchi and colleagues studied linkage to markers on chromosome 17 and obtained significant evidence for linkage to 17q25 (Yamauchi et al. 2000). These findings suggest that several loci are involved in the genetic etiology of moyamoya disease, as predicted by the epidemiological data. Here we report the identification of a novel susceptible locus for moyamoya disease.
Materials and methods
We performed a genome-wide screen of 12 nuclear families with moyamoya disease-affected sib pairs (46 members, 12 sib pairs, two families lacking a paternal sample), who had not been used for genome-wide linkage analysis previously. These samples were generously supplied by the DNA bank holding samples from individuals with moyamoya disease. Seven out of the 12 families had been used to confirm linkage to 3p in the previous study (Ikeda et al. 1999). We were not able to screen other families examined in our previous study because sufficient DNA was not available. No history of intermarriage was evident. Moyamoya disease was diagnosed on the basis of the guidelines for the diagnosis of moyamoya disease established by the Research Committee on Spontaneous Occlusion of the Circle of Willis of the Ministry of Health and Welfare of Japan (Ikezaki et al. 1997). Written informed consent was obtained from all subjects.
DNA was extracted from peripheral blood leukocytes with the standard phenol extraction method. Genotyping was performed with the ABI PRISM Linkage Mapping Set version 2 (Applied Biosystems, Foster City, CA, USA). For higher resolution mapping of chromosome 8, 17 additional markers, D8S1136, D8S530, D8S2324, D8S1757, D8S275, D8S1119, D8S88, GATA8B01, ATA28F12, D8S1699, D8S1772, D8S506, D8S559, D8S546, D8S521, D8S1459, and D8S1703, were used. For higher resolution mapping of chromosome 12, 20 additional markers, D12S1690, D12S77, D12S391, D12S358, D12S320, D12S1728, D12S1715, D12S373, D12S1682, D12S799, D12S1654, GATA91H01, D12S1591, D12S1057, D12S1034, D12S87, D12S1653, D12S1635, D12S1633, and D12S1677, were used. These additional markers were selected from those listed on the publicly available genetic map at the Marshfield Medical Clinic Web site (http://www.marshmed.org/genetics/Map_Markers/maps/indexmap.html). The total number of markers analyzed was 428. The microsatellite markers were amplified by polymerase chain reaction (PCR), and electrophoresis was carried out with an ABI PRISM model 3100 DNA Sequencer equipped with GeneScan software. Analysis was performed with GeneScan and Genotyper software (Applied Biosystems) as described in the manufacturer’s manuals.
Allele frequencies were estimated from the parental chromosomes with the SIB-PAIR program (ver 0.99.9, http://www.qimr.edu.au/davidD/davidd.html/). Information about marker order and position was obtained from the Marshfield Medical Clinic Web site. Linkage analyses were performed with GENEHUNTER 2 software (Kruglyak et al. 1996) and GENEHUNTER-PLUS software (Kong and Cox 1997) for the multipoint and single-point nonparametric analyses, respectively. Allele frequencies estimated from the parental chromosomes were used. We used the nonparametric linkage (NPL) score (Z all) and MLS for multipoint analysis and Zlr and nonparametric LOD statistics for single-point analysis. Transmission disequilibrium test (TDT) was done with the SIB-PAIR program (http://www.qimr.edu.au/davidD/davidd.html).
Linkage results were interpreted empirically by multipoint simulation analysis. We used all families and all genetic markers in the actual analysis to generate 1,000 unlinked replicates with the Simulate program (ftp://linkage.cpmc.columbia.edu/software/simulate) and conducted analyses with the same software (GENEHUNTER ver 2) used in the actual analysis. We calculated the empirical genome-wide significance level as the proportion of replicates for which the NPL or MLS value was greater than that obtained in the real data set. Suggestive and significant evidence for linkage were defined according to published criteria (Lander and Kruglyak 1995) as one random occurrence per genome screen for suggestive evidence and 0.05 times for significant evidence. The simulation procedure suggested that in the absence of linkage, we would observe on average one multipoint MLS and NPL of 1.8 and 2.1, respectively, per genome scan. In the absence of linkage, MLS and NPL values of 3.2 and 2.9, respectively, would have been expected only once in every 20 genome scans, respectively. Thus, MLS values greater than 1.8 and 3.2 and NPLs greater than 2.1 and 2.9 were interpreted as suggestive and significant evidence of linkage, respectively.
Results and discussion
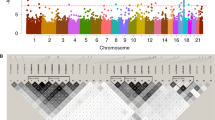
A genome-wide scan with 391 markers revealed several markers on chromosome 8q (MLS=2.8) and 12p (MLS=2.2) with suggestive evidence for linkage. Subsequently, we analyzed linkage with 17 additional markers on 8q and 20 additional markers on 12p. The region near D8S546 on 8q23.1 yielded significant linkage with an MLS of 3.6 and an NPL of 3.3 (Table 1, Fig. 1). Marker D12S1690 on 12p12 achieved a suggestive linkage level with an MLS of 2.3 and an NPL of 2.5. The proportions of identical-by-descent (IBD) sharing (2, 1, 0) were 0.89 for 2, 0.11 for 1, and 0 for 0 at D8S546. The proportions of IBD sharing were 0.77 for 2, 0.23 for 1, and 0 for 0 at D12S1690.
The evidence for linkage of the chromosome 8 has not been reported by other studies. The genome-wide scan in the present study yielded MLS values of 1.7, 1.6, and 1.3 for markers on chromosomes 3p, 6q, and 17q, respectively, where genetic susceptibility loci have been reported previously. However, these markers did not reach a suggestive linkage level, possibly because of the smaller sample size in the present study in comparison with those of the previous studies (Ikeda et al. 1999; Inoue et al. 2000; Yamauchi et al. 2000). The power of this study to detect suggestive linkage was 10% under an autosomal semidominant model with an assumed disease gene frequency of 0.0005 and penetrances of 0.0002, 0.025, and 0.05.
To narrow linkage regions, linkage disequilibrium analysis was done with the SIB-PAIR program for markers in the 8q23 and 12p12 regions. P values for global TDT of 0.008 for D8S546 and 0.003 for D12S1690 were observed. Allele-by-allele TDT revealed that the 160-bp allele of D8S546 was transmitted disproportionately to affected offspring (11 were transmitted and two not transmitted to patients, p=0.01). However, the 160-bp allele was also the most frequently observed allele in 96 chromosomes from randomly selected, apparently healthy Japanese persons with an allele frequency of 0.69. No specific allele of D12S1690 was transmitted in a statistically significant manner to affected offspring. No specific two-locus haplotypes comprising D8S546 or D12S1690 and adjacent markers were present exclusively in patients. Thus, we did not find linkage disequilibrium between the markers examined in this study and moyamoya disease.
Because the pathogenesis of moyamoya disease remains undetermined, candidate genes are hardly selected. Prominent features of moyamoya disease are intimal thickening of the cerebral arterial trunks and abundant angiogenesis for collateral blood supplies. The expression of transforming growth factor-beta1 (TGFβ1) in cultured smooth-muscle cells derived from the superficial temporal arteries and the levels of TGFβ1 in serum of patients with moyamoya disease are significantly higher than those of controls (Hojo et al. 1998). Therefore, the gene encoding TIEG, transforming growth factor-beta-inducible early growth response, located in 8q22.3, is one of the candidates for moyamoya disease. Other candidate genes located in the 8q linkage region include ANGPT1, a secreted ligand for TIE2, a receptor-like tyrosine kinase expressed almost exclusively in endothelial cells and early hematopoietic cells and required for the normal development of vascular structures during embryogenesis; EBAG9, estrogen receptor-binding site-associated antigen, 9; and DD5, a progestin-induced protein (progestin may regulate angiogenesis through vascular endothelial growth factor expression). The findings of the present study will offer an important step toward identification of genes susceptible to moyamoya disease, which will lead to effective treatment and prevention methods.
References
Fukui M, Kono S, Sueishi K, Ikezaki K (2000) Moyamoya disease. Neuropathology 20 [Suppl]:S61–S64
Goto Y, Yonekawa Y (1992) Worldwide distribution of moyamoya disease. Neurol Med Chir (Tokyo) 32:883–886
Hojo M, Hoshimaru M, Miyamoto S, Taki W, Nagata I, Asahi M, Matsuura N, Ishizaki R, Kikuchi H, Hashimoto N (1998) Role of transforming growth factor-beta1 in the pathogenesis of moyamoya disease. J Neurosurg 89:623–629
Ikeda H, Sasaki T, Yoshimoto T, Fukui M, Arinami T (1999) Mapping of a familial moyamoya disease gene to chromosome 3p24.2–p26. Am J Hum Genet 64:533–537
Ikezaki K, Loftus CM (2001) Moyamoya disease. The American Association of Neurological Surgeons, Rolling Meadows
Ikezaki K, Han DH, Kawano T, Kinukawa N, Fukui M (1997) A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke 28:2513–2517
Inoue TK, Ikezaki K, Sasazuki T, Matsushima T, Fukui M (2000) Linkage analysis of moyamoya disease on chromosome 6. J Child Neurol 15:179–182
Kong A, Cox NJ (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188
Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363
Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247
Osawa M, Kanai N, Kawai M, Fukuyama Y (1992) Clinical genetic study on the idiopathic occlusion of the circle of Willis. In: The Research Committee on Spontaneous Occlusion of the Circle of Willis (moyamoya disease) of the Ministry of Health and Welfare Japan: Annual Report Tokyo. Japan: Ministry of Health and Welfare Japan, pp 147–152
Wakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, Kojima M, Lin Y, Ohno Y (1997) Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg 99 [Suppl 2]:S1–S5
Yamauchi T, Houkin K, Tada M, Abe H (1997) Familial occurrence of moyamoya disease. Clin Neurol Neurosurg 99 [Suppl 2]:S162–S167
Yamauchi T, Tada M, Houkin K, Tanaka T, Nakamura Y, Kuroda S, Abe H, Inoue T, Ikezaki K, Matsushima T, Fukui M (2000) Linkage of familial moyamoya disease (spontaneous occlusion of the Circle of Willis) to chromosome 17q25. Stroke 31:930–935
Acknowledgements
This work was supported by a grant from the Research Committee on Spontaneous Occlusion of the Circle of Willis sponsored by the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakurai, K., Horiuchi, Y., Ikeda, H. et al. A novel susceptibility locus for moyamoya disease on chromosome 8q23. J Hum Genet 49, 278–281 (2004). https://doi.org/10.1007/s10038-004-0143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-004-0143-6
Keywords
This article is cited by
-
The Genetic Basis of Moyamoya Disease
Translational Stroke Research (2022)
-
Cerebrovascular disorders associated with genetic lesions
Cellular and Molecular Life Sciences (2019)
-
Moyamoya complicated by thrombotic cerebrovascular accident in a Caucasian woman with collagenous colitis
Neurological Sciences (2018)