Abstract
Mutations in the X-linked gene doublecortex (DCX) result in lissencephaly in males or subcortical laminar heterotopia (double cortex) in females. Recently, an evolutionarily conserved doublecortin (DC) domain important for microtubule binding and microtubule polymerization was defined according to detailed sequence analysis of DCX and DCX-like proteins. Subsequently we cloned a novel human cDNA that contained a DC domain during large-scale DNA sequencing of the human fetal brain cDNA library, and termed it doublecortin-domain-containing 1 (DCDC1). According to a search against the human genome database, DCDC1 was mapped to 11p13. Expression analysis showed that DCDC1 was mainly expressed in adult testis. Furthermore, the expression level of DCDC1 in fetal brain was much higher than in adult brain.
Similar content being viewed by others
Lissencephaly (LIS) is one of the most severe human cerebral cortical malformations (Dobyns and Truwit 1995), which is thought to result from a failure of neuronal migration. Besides the first lissencephaly-associated gene lissencephaly-1 (LIS1 or PAFA1B1) identified on chromosome 17p13, another gene named doublecortex (DCX, or XLIS) was found to be associated with classical type I lissencephaly in humans recently (Portes et al. 1998; Gleeson et al. 1998). Mutations in the X-linked DCX cause gross neocortical disorganization (lissencephaly or "smooth brain") in hemizygous males, whereas heterozygous females show a mosaic phenotype with a normal cortex, as well as a second band of misplaced (heterotopic) neurons beneath the cortex ("double cortex syndrome"). It was found that DCX co-localized with the microtubules, bound microtubules directly, stabilized them and caused bundling (Horesh et al. 1999).
So far, the closest homologue of DCX is a gene named doublecortin and CaM kinase-like 1 (DCAMKL1, also known as KIAA0369) (Omori et al. 1998). DCX and DCAMKL1 share homology throughout the entire DCX amino acid sequence, but DCAMKL1 is twice larger. The unique C terminus of DCAMKL1 contains a domain similar to Ca2+/calmodulin-dependent (CAM) kinases. Both DCX and DCAMKL1 have microtubule binding and polymerization activities, and are co-expressed in migrating neurons in developing brain (Mizuguchi et al. 1999; Lin et al. 2000). These results suggested that DCX and DCAMKL1 might together form a signaling pathway that regulates microtubules in migrating neurons. Other DCX-like genes were also found to be associated with some important inherited diseases. For example, mutations in a retina-specific DCAMKL1-like gene named retinitis pigmentosa 1 (RP1) cause autosomal dominant retinitis pigmentosa (Sullivan et al. 1999).
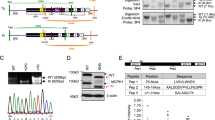
A novel cDNA clone was isolated from a large-scale DNA sequencing of the human fetal brain cDNA library, which was constructed by our laboratory (Xu et al. 2001). Its nucleotide sequence is available from GenBank under accession number AY247970 (Fig. 1a). This 1.7-kb cDNA spans an open reading frame from nucleotide 203 to 1264, encoding a putative 354-amino-acid protein with a predicted molecular mass of 39.8 kDa, and a predicted isoelectric point of 9.40. An in-frame stop codon was found at position 158–160. The presumed initiation codon has a Kozak consensus sequence. And a putative polyadenylation signal, AATAAA, was found near the 3′ end of the sequence. Thus, it was concluded that the coding sequence is complete.
The sequences and domain analysis of DCDC1. a Sequence of the DCDC1 gene. The in-frame codons and polyadenylation signals are underlined. Amino acids are represented below the DNA sequences. The asterisk represents the stop codon. The arrow indicates the doublecortin (DC) domain. b Alignment of the DC domains in DCDC1 and other human DC-domain-containing proteins, including DCX (GenBank accession no. NP_000546; domain I: 48–138, domain II: 175–263), DCAMKL1 (accession no. NP_004725; domain I: 52–142, domain II: 181–269), RP1 (accession no. XP_129368; domain I: 30–117, domain II: 152–236), and RU2S (accession no. AAF23612; domain I: 12–100, domain II 134–221). Alignment was performed by Clustal W algorithms, and amino acids were shaded according to the degree of conservation using GeneDoc (http://www.cris.com/~Ketchup/genedoc.shtml): black (80–100% similarity), and grey (60–80% similarity). The positions of missence mutations in DCX that cause lissencephaly are shown by asterisks
By using BlastP (NCBI web server, http://www.ncbi.nlm.nih.gov/BLAST), the deduced protein was shown to be homologous to some putative proteins (GenBank accession no. XP_158837, XP_242096, XP_158841, XP_034415, BAA96017, XP_289880, BAC26042, XP_283759, XP_230359, AAF08814, and AF194079) and DCX, with similarities from 46% to 81%. Most of their homologous regions located between residues 170 and 246 in the deduced amino acid sequence of the cDNA we cloned. This region was later identified as a doublecortin domain (Bioinformatics Web server, http://www.isrec.isb-sib.ch/software/PFSCAN_form.html) (Fig. 1b). Therefore, we termed this gene doublecortin domain containing 1 (DCDC1), in agreement with the HUGO Nomenclature Committee. The doublecortin (DC) domain is an evolutionarily conserved domain defined according to detailed sequence analysis of DCX and DCX-like proteins (Sapir et al. 2000). In the large majority of patients, missense mutations in DCX fall within the conserved regions, such as Y125H, A71S, R59H/L, D62N, G253D, R192W, and T203R. The domain commonly appears in the N terminus of proteins and consists of two tandemly repeated 80-amino-acid regions. In vitro and in vivo experiments indicated that each repeat alone bound tubulin, while neither repeat was sufficient for co-assembly with microtubules. However, two tandem repeats were sufficient to mediate microtubule polymerization (Taylor et al. 2000). The DC domain of DCDC1 contains only one repeat. This means that DCDC1 might only have microtubule binding activity, but no ability to mediate microtubule polymerization. The absence of a signal peptide (PSORT II server, http://psort.nibb.ac.jp) and a transmembrane domain suggests that DCDC1 is a hydrophilic, intracellular protein. It might also have some other domains with unknown function.
By searching against the human EST database and the human genome database, we found the DCDC1 gene to be represented by five ESTs and two genomic clones (accession nos. NT_030801.7, AL162614.25 and AL137804.7) from chromosome 11p13. Comparison of the cDNA sequence of DCDC1 to the genomic sequence revealed that the DCDC1 gene spanned about 107 kb of genomic DNA and consisted of nine exons. We could thus determine the complete exon-intron structure of the DCDC1 gene (Table 1).
To investigate the expression pattern of DCDC1 in different tissues, we used two human multiple tissue cDNA (MTC; Clontech, Palo Alto, Calif., USA) panels as PCR templates according to the manufacturer's protocol. The DCDC1-specific primer pairs (DCDC1F: 5′-tgggtgatttctgcatttccaactaag-3′; DCDC1R: 5′-ataaacatcggcttcatggggtatatc-3′) were designed to amplify a 0.7-kb fragment. A G3PDH control primer pair included in the panels was used to verify the normalization of the MTC panels. A total of 36 cycles of amplification were performed using rTaq DNA polymerase (TaKaRa, Tokyo, Japan) in a total volume of 50 μl. All the reactions were paused after a total of 27 cycles, 30 cycles, 33 cycles and 36 cycles. Each time, 5-μl samples of each reaction mixture were removed to run on a gel, and the rest put back in the thermal cycler. The cycling conditions were as follows: 2 min at 94 °C, followed by cycles of 30 s at 94 °C, 90 s at 68 °C, with a 5 min 68 °C step to finish. When 27 cycles of amplification was performed, G3PDH (positive control) RT-PCR products were detected in all tissues tested. After 36 cycles the signal was detected in testis, lung, kidney and pancreas. However, the band in testis was much brighter than in either of the other tissues (Fig. 2a). Thus, we concluded that DCDC1 was expressed mostly in testis.
Expression pattern of DCDC1. a Reverse transcription-PCR analysis of human adult tissues cDNA for DCDC1 and G3PDH (positive control). Results of 36 cycles (for DCDC1) and 27 cycles (for G3PDH) of amplification are shown. b Reverse transcription-PCR analysis of human fetal brain and brain tissues cDNA for DCDC1 and G3PDH (positive control). Results of 38 cycles (for DCDC1) and 27 cycles (for G3PDH) are shown
We noticed that DCDC1 cDNA could be isolated from fetal brain but could not be detected in adult brain tissue. We further used the cDNAs of these two tissues from MTC as PCR templates to compare the expression levels in fetal brain and adult brain. Both the primers and the cycling conditions were the same as above. After 38 cycles, it was found that the expression level of DCDC1 in fetal brain was much higher than in adult brain (Fig. 2b). Further study should be made to corroborate DCDC1 as a microtubule-associated protein and to clarify the precise role of DCDC1 gene in testis.
References
Dobyns WB, Truwit CL (1995) Lissencephaly and other malformations of cortical development. Neuropediatrics 23:132–147
Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA (1998) doublecortin, a brain-specific gene mutated in human X-linker lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92:63–72
Horesh D, Sapir T, Francis F, Wolf SG, Caspi M, Elbaum M, Chelly J, Reiner O (1999) Doublecortin, a stabilizer of microtubules. Hum Mol Genet 8:1599–1610
Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA (2000) DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci 20:9152–9161
Mizuguchi M, Qin J, Yamada M, Ikeda K, Takashima S (1999) High expression of doublecortin and KIAA0369 protein in fetal brain suggests their specific role in neuronal migration. Am J Pathol 155:1713–1721
Omori Y, Suzuki M, Ozaki K, Harada Y, Nakamura Y, Takahashi E, Fujiwara T (1998) Expression and chromosomal localization of KIAA0369, a putative kinase structurally related to Doublecortin. J Hum Genet 43:169–177
Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J (1998) A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92:51–61
Sapir T, Horesh D, Caspi M, Atlas R, Burgess HA, Wolf SG, Francis F, Chelly J, Elbaum M, Pietrokovski S, Reiner O (2000) Doublecortin mutations cluster in evolutionarily conserved functional domains. Hum Mol Genet 9:703–712
Sullivan LS, Heckenlively JR, Bowne SJ, Zuo J. Hide WA, Gal A, Denton M, Inglehearn CF, Blanton SH, Daiger SP (1999) Mutations in a novel retina-specific gene cause autosomal dominant retinitis pigmentosa. Nat Genet 22:255–259
Taylor KR, Holzer AK, Bazan JF, Walsh CA, Gleeson JG (2000) Patient mutations in doublecortin define a repeated tubulin-binding domain. J Biol Chem 275:34442–34450
Xu J, Zhou Z, Zeng L, Huang Y, Zhao W, Cheng C, Xu M, Xie Y, Mao Y (2001) Cloning, expression and characterization of a novel human REPS1 gene. Biochem Biophys Acta 1522:118–121
Acknowledgements
This work was supported by the 863 project of P.R. China (grant no. 2001AA221181) and the National Science Foundation of China (30170345).
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Zeng and S. Gu contributed equally to this paper
Rights and permissions
About this article
Cite this article
Zeng, L., Gu, S., Li, Y. et al. Identification of a novel human doublecortin-domain-containing gene (DCDC1) expressed mainly in testis. J Hum Genet 48, 393–396 (2003). https://doi.org/10.1007/s10038-003-0033-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-003-0033-3
Keywords
This article is cited by
-
Transcriptomic differences between fibrotic and non-fibrotic testicular tissue reveal possible key players in Klinefelter syndrome-related testicular fibrosis
Scientific Reports (2022)
-
The genetic architecture of the human thalamus and its overlap with ten common brain disorders
Nature Communications (2021)
-
Comprehensive genomic analysis of Oesophageal Squamous Cell Carcinoma reveals clinical relevance
Scientific Reports (2017)
-
An efficient and flexible test for rare variant effects
European Journal of Human Genetics (2017)
-
Angiopoietin-like gene expression in the mouse uterus during implantation and in response to steroids
Cell and Tissue Research (2012)