Abstract
Purpose
The implantation of non-absorbable meshes is the gold standard technique for ventral hernia (VH) repairs. However, emergency surgeries are often related to contaminated/infected fields, where the implantation of prosthetic materials may not be recommendable. Our aim was to evaluate the results of polyvinylidene fluoride (PVDF) meshes used for contaminated and/or complicated VH repairs in the acute setting.
Methods
We conducted a retrospective analysis of patients with VH who underwent emergency surgery involving PVDF meshes, in a tertiary hospital (from November 2013 to September 2019). We analyzed postoperative complications and 1-year recurrence rates. We evaluated the relationships between contamination grade, mesh placement, infectious complications, and recurrences.
Results
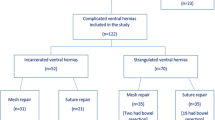
We gathered data on 123 patients; their mean age was 62.3 years, their mean BMI was 31.1 kg/m2, and their mean CeDAR index was 51.6. 96.4% of patients had a grade 2–3 ventral hernia according to the Rosen index. The mean defect width was 8 cm (IQR 2–18). 93 cases (75.6%) were described as contaminated or dirty surgeries. A PVDF mesh was placed using an IPOM technique in 56.3% of cases, and via interposition location in 39.9%. The one-month recurrence rate was 5.7% and recurrence after one year was 19.1%. The overall mortality rate was 27.6%. Risk of recurrence was related to patients with a Rosen score over 2 (p < 0.001), as well as with postoperative SSI (p = 0.045). Higher recurrence rates were not related to PVDF mesh placement.
Conclusion
The use of PVDF meshes for emergency VH repairs in contaminated surgeries seems safe and useful, with reasonable recurrence rates, and acceptable infectious complication rates, similar to those published in the literature.

Similar content being viewed by others
References
Jairam AP, Timmermans L, Eker HH et al (2017) Prevention of ventral hernia with prophylactic onlay and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2-year follow-up of a multicentre, double-blind, randomised controlled trial. Lancet 390:567–576
Ahn BK (2012) Risk factors for ventral hernia and parastomal hernia after colorectal surgery. J Korean Soc Coloproctol 28:280–281
Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of ventral hernia. Ann Surg 240:578–583
Lopez-Cano M, Martin-Dominguez LA, Pereira JA, Armengol-Carrasco M, Garcia-Alamino JM (2018) Balancing mesh-related complications and benefits in primary ventral and ventral hernia surgery. A meta-analysis and trial sequential analysis. PLoS ONE 13:e0197813
Klink CD, Junge K, Binnebösel M et al (2011) Comparison of long-term biocompatibility of PVDF and PP meshes. J Invest Surg 24:292–299
Fitzgerald JF, Kumar AS (2014) Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin Colon Rectal Surg 27:140–148
Sahoo S, Haskins IN, Huang LC et al (2017) Early wound morbidity after open VENTRAL hernia repair with biosynthetic or polypropylene mesh. J Am Coll Surg 225:472–480.e1
Junge K, Binnebösel M, Rosch R et al (2009) Adhesion formation of a polyvinylidenfluoride/polypropylene mesh for intra-abdominal placement in a rodent animal model. Surg Endosc 23:327–333
Laroche G, Marois Y, Schwarz E et al (1995) Polyvinylidene fluoride monofilament sutures: can they be used safely for long-term anastomoses in the thoracic aorta? Artif Organs 19:1190–1199
Klinge U, Klosterhalfen B, Ottinger AP, Junge K, Schumpelick V (2002) PVDF as a new polymer for the construction of surgical meshes. Biomaterials 23:3487–3493
Augenstein VA, Colavita PD, Wormer BA, Walters AL, Bradley JF, Lincourt AE et al (2015) CeDAR: carolinas equation for determining associated risks. J Am Coll Surg 1(221):S65–S66
Rosen MJ, Bauer JJ, Harmaty M et al (2017) Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg 265:205–211
Garner JS (1986) CDC guideline for prevention of surgical wound infections, 1985. Supersedes guideline for prevention of surgical wound infections published in 1982. (Originally published in November 1985). Revised. Infect Control 7:193–200
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Birolini C, de Miranda JS, Tanaka EY, Utiyama EM, Rasslan S, Birolini D (2020) The use of synthetic mesh in contaminated and infected abdominal wall repairs: challenging the dogma—A long-term prospective clinical trial. Hernia 24(2):307–323
Breuing K, Butler CE, Ferzoco S et al (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148:544–558
Itani KM, Rosen M, Vargo D et al (2012) Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 152:498–505
Rosen MJ, Bauer JJ, Harmaty M et al (2017) Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA Study. Ann Surg 265:205–211
Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ (2013) Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg 217:991–998
Atema JJ, De Vries FE, Boermeester MA (2016) Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am J Surg 212:982–995.e1
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27:97–132
Choi JJ, Palaniappa NC, Dallas KB, Rudich TB, Colon MJ, Divino CM (2012) Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg 255:176–180
De Simone B, Birindelli A, Ansaloni L, Sartelli M, Coccolini F, Di Saverio S, Annessi V, Amico F, Catena F (2020) Emergency repair of complicated abdominal wall hernias: WSES guidelines. Hernia 24(2):359–368
Pereira JA, López-Cano M, Hernández-Granados P, Feliu X, en representación del grupo EVEREG (2016) Resultados iniciales del Registro Español de Hernia Incisional. Cir Esp 94:595–602
Berger D, Bientzle M (2009) Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! A prospective, observational study with 344 patients. Hernia 13:167–172
Junge K, Binnebösel M, Rosch R, Jansen M, Kämmer D, Otto J, Schumpelick V, Klinge U (2009) Adhesion formation of a polyvinylidenfluoride/polypropylene mesh for intra-abdominal placement in a rodent animal model. Surg Endosc 23:327–333
Mary C, Marois Y, King MW, Laroche G, Douville Y, Martin L, Guidoin R (1998) Comparison of the in vivo behavior of polyvinylidene fluoride and polypropylene sutures used in vascular surgery. ASAIO J 44:199–206
Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ (2013) Outcomes of synthetic mesh in contaminated VENTRAL hernia repairs. J Am Coll Surg 217:991–998
Chamieh J, Tan WH, Ramirez R, Nohra E, Apakama C, Symons W (2017) Synthetic versus biologic mesh in single-stage repair of complex abdominal wall defects in a contaminated field. Surg Infect (Larchmt) 18:112–118
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257:991–996
Poulose BK, Shelton J, Phillips S et al (2012) Epidemiology and cost of VENTRAL hernia repair: making the case for hernia research. Hernia 16:179–183
Köckerling F, Alam NN, Antoniou SA et al (2018) What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia 22:249–269
HolIhan JL, Hannon C, Goodenough C et al (2017) VENTRAL hernia repair: a meta-analysis of randomized controlled trials. Surg Infect (Larchmt) 18:647–658
Stabilini C, Cavallaro G, Dolce P, Capoccia Giovannini S, Corcione F, Frascio M, Sodo M, Merola G, Bracale U (2019) Pooled data analysis of primary ventral (PVH) and incisional hernia (IH) repair is no more acceptable: results of a systematic review and metanalysis of current literature. Hernia 23(5):831–845
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not declare any conflict of interest or sources of funding for the present work. The authors also declare that the present work is not based on a previous communication to a society or meeting.
Ethical approval
Ethical approval for this retrospective study was not required.
Human and animal rights
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
For this type of study, formal consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Arteaga, A., Tallón-Aguilar, L., Tinoco-González, J. et al. Use of polyvinylidene fluoride (PVDF) meshes for ventral hernia repair in emergency surgery. Hernia 25, 99–106 (2021). https://doi.org/10.1007/s10029-020-02209-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-020-02209-3