Abstract
Purpose
Biologic prostheses are designed to support tissue regeneration rather than just result in a strong scar plate, as is the case with synthetic mesh. It is not known if these newer materials will result in earlier return to normal activities and/or less post-herniorrhaphy groin pain.
Method/study design
A prospective, randomized, controlled, third-party-blinded multicenter trial was designed to compare the use of a non-cross linked porcine dermis biologic graft [StratticeTM Reconstructive Tissue Matrix (RTM), LifeCell, Branchburg, NJ] versus light weight, large pore polypropylene mesh (UltraProTM, Ethicon, Somerville, NJ). The study design called for recruitment of 170 men. These men are being followed for a minimum of 2 years. The primary aim of this study is to compare the safety and effectiveness of the two materials in a Lichtenstein inguinal hernia repair as measured by resumption of activities of daily living. Secondary outcomes include chronic pain, postoperative complications and the incidence of re-herniation at 12 and 24 months.
Results
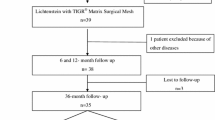
This paper discusses the study design, patient recruitment and the current status of the clinical trial. The study involves nine medical centers, all with extensive experience in hernia repair. After 24 months of enrollment, 172 men were randomized and recruitment was then closed. All patients underwent elective repair of primary unilateral inguinal hernias as an outpatient operation. Follow up data are being collected. Data analyses are scheduled at 3, 12, and 24 months postoperatively.
Conclusion
We report the design of a multi-center, third-party blinded, randomized clinical trial comparing a new surgical device with existing technology in the repair of inguinal hernias. We believe this investigator-designed and conducted trial could serve as a model for similar trials examining surgical devices performed in collaboration with industry.

Similar content being viewed by others
Abbreviations
- FDA:
-
Food and Drug Administration
- AAS:
-
Activities Assessment Scale
- PRCT:
-
Prospective, randomized, controlled trial
References
Maisel WH (2004) Medical device regulation: an introduction for the practicing physician. Ann Intern Med 140:296–302
Rutkow IM (2003) Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am 83:1045–1051 (v–vi)
Grant AM, EU Hernia Trialists Collaboration (2002) Open mesh versus non-mesh repair of groin hernia: meta-analysis of randomised trials based on individual patient data. Hernia 6:130–136
Conze J, Kingsnorth AN, Flament JB, Simmermacher R, Arlt G, Langer C, Schippers E, Hartley M, Schumpelick V (2005) Randomized clinical trial comparing lightweight composite mesh with polyester or polypropylene mesh for incisional hernia repair. Br J Surg 92:1488–1493
Bringman S, Wollert S, Osterberg J, Smedberg S, Granlund H, Heikkinen TJ (2006) Three-year results of a randomized clinical trial of lightweight or standard polypropylene mesh in Lichtenstein repair of primary inguinal hernia. Br J Surg 93:1056–1059
Cornwell KG, Landsman A, James KS (2009) Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 26:507–523
Bachman S, Ramshaw B (2008) Prosthetic material in ventral hernia repair: how do I choose? Surg Clin North Am 88:101–112 (ix)
Smart N, Immanuel A, Mercer-Jones M (2007) Laparoscopic repair of a Littre’s hernia with porcine dermal collagen implant (Permacol). Hernia 11:373–376
Albo D, Awad SS, Berger DH, Bellows CF (2006) Decellularized human cadaveric dermis provides a safe alternative for primary inguinal hernia repair in contaminated surgical fields. Am J Surg 192:e12–e17
Ansaloni L, Catena F, Coccolini F, Gazzotti F, D’Alessandro L, Pinna AD (2009) Inguinal hernia repair with porcine small intestine submucosa: 3-year follow-up results of a randomized controlled trial of Lichtenstein’s repair with polypropylene mesh versus Surgisis Inguinal Hernia Matrix. Am J Surg 3:303–312
Edelman DS, Hodde JP (2006) Bioactive prosthetic material for treatment of hernias. Surg Technol Int 15:104–108
Cingi A, Manukyan MN, Gulluoglu BM, Barlas A, Yegen C, Yahn R, Yilmaz N, Aktan AO (2005) Use of resterilized polypropylene mesh in inguinal hernia repair: a prospective, randomized study. J Am Coll Surg 201:834–840
Cook Medical (2008) Cost–benefit analysis: ventral hernia repair. Cook Medical Incorporated, Bloomington
Kaleya RN, Thomas R (2005) Use of a global economic model to analyze the cost-benefit of Alloderm in Ventral Hernia Repair. LifeCell Corporation, Branchburg, NJ
Decision Resources, Inc. (2007) SAGE sourcebook of modern biomedical devices: business environments in a global market. SAGE, Thousand Oaks, CA
McCarthy M Jr, Jonasson O, Chang CH, Pickard AS, Giobbie-Hurder A, Gibbs J, Edelman P, Fitzgibbons R, Neumayer L (2005) Assessment of patient functional status after surgery. J Am Coll Surg 201:171–178
Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS (2004) Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 20:309–318
Wong DL, Hockenberry-Eaton M, Wilson D, Winkelstein ML, Schwartz P (2001) Wong’s essentials of pediatric nursing (6th edn). Mosby, St Louis, p 1301
Berg DM (1993) Pain assessment tool preference among adults. Indiana University School of Nursing, Indianapolis, Indiana
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Hospital Infection Control Practices Advisory Committee (1999) Guideline for Prevention of Surgical Site Infection. Centers for Disease Control and Prevention (CDC). Am J Infect Control 27:97–132
Acknowledgments
This trial was developed and funded in cooperation with LifeCell, Inc., (Branchburg, NJ). The authors have the following industry relations: C.B. and W.H. receive honoraria as consultants and lecturers for LifeCell Corporation. P.S. serves as a consultant to Allergan Medical, Ethicon Inc, Ethicon Endosurgery, LifeCell, and TransEnterix. R.F. serves as an ad hoc consultant to LifeCell and Baxter corporations, and has grant support from LifeCell (for the present study) and Biomerix Corporation (for a Phase II Trial to Determine Efficacy of a Polycarbonate, Polyurethane Biomaterial for Repair of an Inguinal Hernia), and a royalty arrangement with Cook Critical Care (for the Fitzgibbons Jenkins Catheter, a common bile duct catheter).
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: This trial has been registered at http://www.clinicaltrials.gov/. The trial registration number is NCT00681291.
Rights and permissions
About this article
Cite this article
Bellows, C.F., Shadduck, P.P., Helton, W.S. et al. The design of an industry-sponsored randomized controlled trial to compare synthetic mesh versus biologic mesh for inguinal hernia repair. Hernia 15, 325–332 (2011). https://doi.org/10.1007/s10029-010-0773-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-010-0773-x