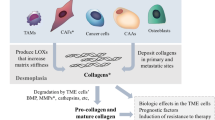
Abstract
Collagen types I, II, and III are the most abundant extracellular matrix (ECM) proteins. Collagenase is a member of the matrix metalloproteinase (MMP) family of enzymes, and is the principal enzyme involved with collagen degradation. Cellular–ECM interactions are vitally important to tissue structure and function. In this review, we summarize recent work that highlights the role of collagenase in ECM remodeling and repair, and further report that alterations of collagenase expression, function, and/or regulation are found in many diverse disease states, including aortic aneurysms, tumor invasiveness and their metastases, and hernias. Collagenase is intimately involved in many surgical diseases, and represents a potential target for therapy.
Similar content being viewed by others
References
Overall CM, Lopez-Otin C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2(9):657–672
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, Maune S (2006) Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck 28(1):31–39
Malemud CJ (2006) Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci 11:1696–1701
Ala-aho R, Kahari VM (2005) Collagenases in cancer. Biochimie 87(3–4):273–286
Baker AH, Edwards DR, Murphy G (2002) Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115(Pt 19):3719–3727
Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477(1–2):267–283
DeClerck YA, Yean TD, Lee Y, Tomich JM, Langley KE (1993) Characterization of the functional domain of tissue inhibitor of metalloproteinases-2 (TIMP-2). Biochem J 289(Pt 1):65–69
Madlener M, Parks WC, Werner S (1998) Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res 242(1):201–210
Saarialho-Kere UK (1998) Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res 290(Suppl):S47–S54
Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, Saarialho-Kere UK (1996) Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 135(1):52–59
Mauviel A (1993) Cytokine regulation of metalloproteinase gene expression. J Cell Biochem 53(4):288–295
Lobmann R, Schultz G, Lehnert H (2005) Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 28(2):461–471
Wirl G, Frick J (1979) Collagenase—a marker enzyme in human bladder cancer? Urol Res 7(2):103–108
McGowan KA, Bauer EA, Smith LT (1994) Localization of type I human skin collagenase in developing embryonic and fetal skin. J Invest Dermatol 102(6):951–957
Ravanti L, Kahari VM (2000) Matrix metalloproteinases in wound repair (review). Int J Mol Med 6(4):391–407
Hasty KA, Hibbs MS, Kang AH, Mainardi CL (1986) Secreted forms of human neutrophil collagenase. J Biol Chem 261(12):5645–5650
Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL (1990) Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem 265(20):11421–11424
Cole AA, Chubinskaya S, Schumacher B, Huch K, Szabo G, Yao J, Mikecz K, Hasty KA, Kuettner KE (1996) Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem 271(18):11023–11026
Hanemaaijer R, Sorsa T, Konttinen YT, Ding Y, Sutinen M, Visser H, van Hinsbergh VW, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T (1997) Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem 272(50):31504–31509
Prikk K, Maisi P, Pirila E, Sepper R, Salo T, Wahlgren J, Sorsa T (2001) In vivo collagenase-2 (MMP-8) expression by human bronchial epithelial cells and monocytes/macrophages in bronchiectasis. J Pathol 194(2):232–238
Gross J, Lapiere CM (1962) Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA 48(6):1014–1022
Knauper V, Cowell S, Smith B Lopez-Otin C, O’Shea M, Morris H, Zardi L, Murphy G (1997) The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem 272(12):7608–7616
Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM (1999) Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J 340(Pt 1):171–181
Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ (1996) Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett 380(1–2):17–20
Thompson RW (2002) Reflections on the pathogenesis of abdominal aortic aneurysms. Cardiovasc Surg 10(4):389–394
Dobrin PB, Mrkvicka R (1994) Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg 2(4):484–488
Busuttil RW, Abou-Zamzam AM, Machleder HI (1980) Collagenase activity of the human aorta. A comparison of patients with and without abdominal aortic aneurysms. Arch Surg 115(11):1373–1378
Busuttil RW, Rinderbriecht H, Flesher A, Carmack C (1982) Elastase activity: the role of elastase in aortic aneurysm formation. J Surg Res 32(3):214–217
Webster MW, McAuley CE, Steed DL, Miller DD, Evans CH (1991) Collagen stability and collagenolytic activity in the normal and aneurysmal human abdominal aorta. Am J Surg 161(6):635–638
Menashi S, Campa JS, Greenhalgh RM, Powell JT (1987) Collagen in abdominal aortic aneurysm: typing, content, and degradation. J Vasc Surg 6(6):578–582
Zarins CK, Runyon-Hass A, Zatina MA, Lu CT, Glagov S (1986) Increased collagenase activity in early aneurysmal dilatation. J Vasc Surg 3(2):238–248
Vine N, Powell JT (1991) Metalloproteinases in degenerative aortic disease. Clin Sci (Lond) 81(2):233–239
Irizarry E, Newman KM, Gandhi RH, Nackman GB, Halpern V, Wishner S, Scholes JV, Tilson MD (1993) Demonstration of interstitial collagenase in abdominal aortic aneurysm disease. J Surg Res 54(6):571–574
Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT (1995) Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 15(8):1145–1151
Knox JB, Sukhova GK, Whittemore AD, Libby P (1997) Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 95(1):205–212
Mao D, Lee JK, VanVickle SJ, Thompson RW (1999) Expression of collagenase-3 (MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture. Biochem Biophys Res Commun 261(3):904–910
Panek B, Gacko M, Palka J (2004) Metalloproteinases, insulin-like growth factor-I and its binding proteins in aortic aneurysm. Int J Exp Pathol 85(3):159–164
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL (1996) Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell 87(4):709–719
Parks WC (1999) Matrix metalloproteinases in repair. Wound Repair Regen 7(6):423–432
Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 137(6):1445–1457
Inoue M, Kratz G, Haegerstrand A, Stahle-Backdahl M (1995) Collagenase expression is rapidly induced in wound-edge keratinocytes after acute injury in human skin, persists during healing, and stops at re-epithelialization. J Invest Dermatol 104(4):479–483
Vaalamo M, Mattila L, Johansson N, Kariniemi AL, Karjalainen-Lindsberg ML, Kahari VM, Saarialho-Kere U (1997) Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol 109(1):96–101
Kahari VM, Saarialho-Kere U (1997) Matrix metalloproteinases in skin. Exp Dermatol 6(5):199–213
Welgus HG, Jeffrey JJ, Eisen AZ (1981) The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem 256(18):9511–9515
Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, Lopez-Otin C (1994) Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 269(24):16766–16773
Le AD, Zhang Q, Wu Y, Messadi DV, Akhondzadeh A, Nguyen AL, Aghaloo TL, Kelly AP, Bertolami CN (2004) Elevated vascular endothelial growth factor in keloids: relevance to tissue fibrosis. Cells Tissues Organs 176(1–3):87–94
Neely AN, Clendening CE, Gardner J, Greenhalgh DG, Warden GD (1999) Gelatinase activity in keloids and hypertrophic scars. Wound Repair Regen 7(3):166–171
Arakawa M, Hatamochi A, Mori Y, Mori K, Ueki H, Moriguchi T (1996) Reduced collagenase gene expression in fibroblasts from hypertrophic scar tissue. Br J Dermatol 134(5):863–868
Uchida G, Yoshimura K, Kitano Y, Okazaki M, Harii K (2003) Tretinoin reverses upregulation of matrix metalloproteinase-13 in human keloid-derived fibroblasts. Exp Dermatol 12(Suppl 2):35–42
Fujiwara M, Muragaki Y, Ooshima A (2005) Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol 153(2):295–300
Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW (1995) Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 104(2):236–240
Vaalamo M, Leivo T, Saarialho-Kere U (1999) Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol 30(7):795–802
Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G (1999) Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 7(6):442–452
Armstrong DG, Jude EB (2002) The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc 92(1):12–18
Cullen B, Smith R, McCulloch E, Silcock D, Morrison L (2002) Mechanism of action of Promogran, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 10(1):16–25
Veves A, Sheehan P, Pham HT (2002) A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 137(7):822–827
Vin F, Teot L, Meaume S (2002) The healing properties of Promogran in venous leg ulcers. J Wound Care 11(9):335–341
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120(3):303–313
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM (2000) Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 18(5):1135–1149
Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH (1996) Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol 149(1):273–282
Moilanen M, Pirila E, Grenman R, Sorsa T, Salo T (2002) Expression and regulation of collagenase-2 (MMP-8) in head and neck squamous cell carcinomas. J Pathol 197(1):72–81
Stadelmann WK, Digenis AG, Tobin GR (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176(2A Suppl):26S–38S
Johansson N, Airola K, Grenman R, Kariniemi AL, Saarialho-Kere U, Kahari VM (1997) Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol 151(2):499–508
Cazorla M, Hernandez L, Nadal A, Balbin M, Lopez JM, Vizoso F, Fernandez PL, Iwata K, Cardesa A, Lopez-Otin C, Campo E (1998) Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol 186(2):144–150
Yamamoto A, Yano S, Shiraga M, Ogawa H, Goto H, Miki T, Zhang H, Sone S (2003) A third-generation matrix metalloproteinase (MMP) inhibitor (ONO-4817) combined with docetaxel suppresses progression of lung micrometastasis of MMP-expressing tumor cells in nude mice. Int J Cancer 103(6):822–828
Zucker S, Mirza H, Conner CE, Lorenz AF, Drews MH, Bahou WF, Jesty J (1998) Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer 75(5):780–786
Libby P, Aikawa M (2002) Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med 8(11):1257–1262
Rutkow IM, Robbins AW (1993) Demographic, classificatory, and socioeconomic aspects of hernia repair in the United States. Surg Clin North Am 73(3):413–426
Tilstra DJ, Byers PH (1994) Molecular basis of hereditary disorders of connective tissue. Annu Rev Med 45:149–163
Krieg T, Hein R, Hatamochi A, Aumailley M (1988) Molecular and clinical aspects of connective tissue. Eur J Clin Invest 18(2):105–123
Lehnert B, Wadouh F (1992) High coincidence of inguinal hernias and abdominal aortic aneurysms. Ann Vasc Surg 6(2):134–137
Zheng H, Si Z, Kasperk R, Bhardwaj RS, Schumpelick V, Klinge U, Klosterhalfen B (2002) Recurrent inguinal hernia: disease of the collagen matrix? World J Surg 26(4):401–408
Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B (2000) Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res 32(1):43–48
Rosch R, Klinge U, Si Z, Junge K, Klosterhalfen B, Schumpelick V (2002) A role for the collagen I/III and MMP-1/-13 genes in primary inguinal hernia? BMC Med Genet 3:2
Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B (2001) Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg 14(1):47–54
Klinge U, Zheng H, Si ZY, Schumpelick V, Bhardwaj R, Klosterhalfen B (1999) Synthesis of type I and III collagen, expression of fibronectin and matrix metalloproteinases-1 and -13 in hernial sac of patients with inguinal hernia. Int J Surg Investig 1(3):219–227
Klinge U, Zheng H, Si Z, Schumpelick V, Bhardwaj RS, Muys L, Klosterhalfen B (1999) Expression of the extracellular matrix proteins collagen I, collagen III and fibronectin and matrix metalloproteinase-1 and -13 in the skin of patients with inguinal hernia. Eur Surg Res 31(6):480–490
Friedman DW, Boyd CD, Norton P, Greco RS, Boyarsky AH, Mackenzie JW, Deak SB (1993) Increases in type III collagen gene expression and protein synthesis in patients with inguinal hernias. Ann Surg 218(6):754–760
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donahue, T.R., Hiatt, J.R. & Busuttil, R.W. Collagenase and surgical disease. Hernia 10, 478–485 (2006). https://doi.org/10.1007/s10029-006-0146-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-006-0146-7