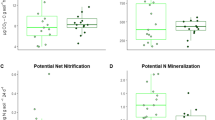
Abstract
Carbon (C) fluxes among different components of plant growth are important to forest ecosystem C cycling and are strongly influenced by species composition and resource availability. Although mycorrhizal fungi are crucial for nutrient acquisition and can receive a large fraction of annual net primary production, most studies do not explicitly include carbon flux to mycorrhizal fungi in ecosystem C budgets. We measured annual production of plant components (foliage, wood, fine roots) and mycorrhizal fungi across temperate forest stands varying in species composition. Production of mycorrhizal fungi was estimated using both mass balance and isotopic techniques. Total plant production varied from about 600 g C m−2 y−1 in nearly pure deciduous broadleaf stands down to about 300 g C m−2 y−1 in conifer-dominated stands. In contrast, the production of mycorrhizal fungi was highest in conifer-dominated stands, varying from less than 25 g C m−2 y−1 in deciduous broadleaf stands to more than 175 g C m−2 y−1 in nearly pure conifer stands. Isotopic data indicated that both tree species composition and ecosystem nitrogen (N) availability influenced rates of fungal production. The large investment in mycorrhizal fungi in low-N, conifer-dominated stands demonstrated that a full accounting of ecosystem carbon fluxes to plant and fungal components may help resolve current discrepancies observed in broadscale forest carbon budgets, especially across forest types.







Similar content being viewed by others
References
Adams MB, Loughry LH, Plaugher LL. 2010. Experimental forests and ranges of the USDA Forest Service. Northeastern Research Station http://ir.library.oregonstate.edu/xmlui/handle/1957/17290. Last accessed 18/09/2017
Addo-Danso SD, Prescott CE, Smith AR. 2016. Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. Forest Ecology and Management 359:332–51.
Aguilar-Trigueros CA, Powell JR, Anderson IC, Antonovics J, Rillig MC. 2014. Ecological understanding of root-infecting fungi using trait-based approaches. Trends in Plant Science 19:432–8.
Allen MF, Allen EB, Lansing JL, Pregitzer KS, Hendrick RL, Ruess RW, Collins SL. 2010. Responses to chronic N fertilization of ectomycorrhizal piñon but not arbuscular mycorrhizal juniper in a piñon-juniper woodland. Journal of Arid Environments 74:1170–6.
Allen MF, Kitajima K. 2014. Net primary production of ectomycorrhizas in a California forest. Functional Ecology 10:81–90.
Amundson R, Austin AT, Schuur EaG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT. 2003. Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem Cycles 17:1031.
Averill C, Finzi A. 2011. Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem δ15 N. Ecology 92:883–91.
Bond-Lamberty B, Thomson A. 2014. A global database of soil respiration data, Version 3.0. Data set Available on-line [http://daac.ornl.gov] from Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, USA http://dx.doi.org/103334/ORNLDAAC/1235. http://www.biogeosciences-discuss.net/7/1321/2010/. Last accessed 24/01/2018
Bradford J, Weishampel P, Smith M-L, Kolka R, Birdsey RA, Ollinger SV, Ryan MG. 2009. Detrital carbon pools in temperate forests: magnitude and potential for landscape-scale assessment. Can J For Res 39:802–13.
Brzostek ER, Rebel KT, Smith KR, Phillips RP. 2017. Chapter 26—Integrating Mycorrhizas Into Global Scale Models: A Journey Toward Relevance in the Earth’s Climate System. In: Johnson NC, Gehring C, Jansa J, editors. Mycorrhizal Mediation of Soil. Elsevier. pp 479–99. http://www.sciencedirect.com/science/article/pii/B9780128043127000267. Last accessed 11/02/2019
Castellano MA, Stephens RB. 2017. Elaphomyces species (Elaphomycetaceae, Eurotiales) from Bartlett Experimental Forest, New Hampshire, USA. IMA Fungus 8:49–63.
Chagnon P-L, Bradley RL, Maherali H, Klironomos JN. 2013. A trait-based framework to understand life history of mycorrhizal fungi. Trends in Plant Science 18:484–91.
Chapin FSIII, McFarland J, David McGuire A, Euskirchen ES, Ruess RW, Kielland K. 2009. The changing global carbon cycle: linking plant–soil carbon dynamics to global consequences. Journal of Ecology 97:840–50.
Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD. 2015. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytologist 205:1525–36.
Comas LH, Eissenstat DM. 2009. Patterns in root trait variation among 25 co-existing North American forest species. New Phytologist 182:919–28.
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Peñuelas J, Reich PB, Schuur EAG, Stock WD, Templer PH, Virginia RA, Welker JM, Wright IJ. 2009. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytologist 183:980–92.
Davidson EA, Savage K, Bolstad P, Clark DA, Curtis PS, Ellsworth DS, Hanson PJ, Law BE, Luo Y, Pregitzer KS, Randolph JC, Zak D. 2002. Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements. Agricultural and Forest Meteorology 113:39–51.
Dybzinski R, Farrior C, Wolf A, Reich PB, Pacala SW. 2011. Evolutionarily Stable Strategy Carbon Allocation to Foliage, Wood, and Fine Roots in Trees Competing for Light and Nitrogen: An Analytically Tractable, Individual-Based Model and Quantitative Comparisons to Data. The American Naturalist 177:153–66.
Eissenstat DM, Yanai RD. 1997. The Ecology of Root Lifespan. Advances in Ecological Research 27:1–60.
Ekblad A, Wallander H, Godbold DL, Cruz C, Johnson D, Baldrian P, Björk RG, Epron D, Kieliszewska-Rokicka B, Kjøller R, Kraigher H, Matzner E, Neumann J, Plassard C. 2013. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil 366:1–27.
Evans RD. 2001. Physiological mechanisms influencing plant nitrogen isotope composition. Trends in Plant Science 6:121–6.
Fahey TJ, Siccama TG, Driscoll CT, Likens GE, Campbell J, Johnson CE, Battles JJ, Aber JD, Cole JJ, Fisk MC, Groffman PM, Hamburg SP, Holmes RT, Schwarz PA, Yanai RD. 2005. The Biogeochemistry of Carbon at Hubbard Brook. Biogeochemistry 75:109–76.
Fernandez CW, Kennedy PG. 2016. Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytologist 209:1382–94.
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP. 2015. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Change Biol 21:2082–94.
Fogel R, Hunt G. 1983. Contribution of mycorrhizae and soil fungi to nutrient cycling in a Douglas-fir ecosystem. Can J For Res 13:219–32.
Fry EL, De Long JR, Álvarez Garrido L, Alvarez N, Carrillo Y, Castañeda-Gómez L, Chomel M, Dondini M, Drake JE, Hasegawa S, Hortal S, Jackson BG, Jiang M, Lavallee JM, Medlyn BE, Rhymes J, Singh BK, Smith P, Anderson IC, Bardgett RD, Baggs EM, Johnson D. 2019. Using plant, microbe, and soil fauna traits to improve the predictive power of biogeochemical models. Methods in Ecology and Evolution 10:146–57.
Giardina CP, Ryan MG. 2002. Total Belowground Carbon Allocation in a Fast-growing Eucalyptus Plantation Estimated Using a Carbon Balance Approach. Ecosystems 5:487–99.
Gill AL, Finzi AC. 2016. Belowground carbon flux links biogeochemical cycles and resource-use efficiency at the global scale. Ecol Lett 19:1419–28.
Godbold DL, Hoosbeek MR, Lukac M, Cotrufo MF, Janssens IA, Ceulemans R, Polle A, Velthorst EJ, Scarascia-Mugnozza G, Angelis PD, Miglietta F, Peressotti A. 2006. Mycorrhizal Hyphal Turnover as a Dominant Process for Carbon Input into Soil Organic Matter. Plant Soil 281:15–24.
Gower ST, Krankina O, Olson RJ, Apps M, Linder S, Wang C. 2001. Net Primary Production and Carbon Allocation Patterns of Boreal Forest Ecosystems. Ecological Applications 11:1395–411.
Heijden MGAVD, Bardgett RD, Straalen NMV. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11:296–310.
Hendricks JJ, Mitchell RJ, Kuehn KA, Pecot SD, Sims SE. 2006. Measuring external mycelia production of ectomycorrhizal fungi in the field: the soil matrix matters. New Phytologist 171:179–86.
Hobbie EA. 2006. Carbon Allocation to Ectomycorrhizal Fungi Correlates with Belowground Allocation in Culture Studies. Ecology 87:563–9.
Hobbie EA, Colpaert JV. 2003. Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytologist 157:115–26.
Hobbie EA, Hobbie JE. 2008. Natural Abundance of 15 N in Nitrogen-Limited Forests and Tundra Can Estimate Nitrogen Cycling Through Mycorrhizal Fungi: A Review. Ecosystems 11:815.
Hobbie EA, Högberg P. 2012. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196:367–82.
Hobbie EA, Ouimette AP. 2009. Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–71.
Hobbie EA, van Diepen LTA, Lilleskov EA, Ouimette AP, Finzi AC, Hofmockel KS. 2014. Fungal functioning in a pine forest: evidence from a 15 N-labeled global change experiment. New Phytol 201:1431–9.
Hollinger DY. 2008. Defining a Landscape-Scale Monitoring Tier for the North American Carbon Program. In: Hoover CM, editor. Field Measurements for Forest Carbon Monitoring. Springer Netherlands. pp 3–16. http://link.springer.com/chapter/10.1007/978-1-4020-8506-2_1. Last accessed 14/11/2016
Kong D, Ma C, Zhang Q, Li L, Chen X, Zeng H, Guo D. 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol 203:863–72.
Litton CM, Giardina CP. 2008. Below-ground carbon flux and partitioning: global patterns and response to temperature. Functional Ecology 22:941–54.
Litton CM, Raich JW, Ryan MG. 2007. Carbon allocation in forest ecosystems. Global Change Biology 13:2089–109.
Luyssaert S, Inglima I, Jung M, Richardson AD, Reichstein M, Papale D, Piao SL, Schulze E-D, Wingate L, Matteucci G, Aragao L, Aubinet M, Beer C, Bernhofer C, Black KG, Bonal D, Bonnefond J-M, Chambers J, Ciais P, Cook B, Davis KJ, Dolman AJ, Gielen B, Goulden M, Grace J, Granier A, Grelle A, Griffis T, Grünwald T, Guidolotti G, Hanson PJ, Harding R, Hollinger DY, Hutyra LR, Kolari P, Kruijt B, Kutsch W, Lagergren F, Laurila T, Law BE, Le Maire G, Lindroth A, Loustau D, Malhi Y, Mateus J, Migliavacca M, Misson L, Montagnani L, Moncrieff J, Moors E, Munger JW, Nikinmaa E, Ollinger SV, Pita G, Rebmann C, Roupsard O, Saigusa N, Sanz MJ, Seufert G, Sierra C, Smith M-L, Tang J, Valentini R, Vesala T, Janssens IA. 2007. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Global Change Biology 13:2509–37.
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM. 2012. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytologist 195:823–31.
McDowell NG, Balster NJ, Marshall JD. 2001. Belowground carbon allocation of Rocky Mountain Douglas-fir. Can J For Res 31:1425–36.
Neumann J, Matzner E. 2013. Biomass of extramatrical ectomycorrhizal mycelium and fine roots in a young Norway spruce stand — a study using ingrowth bags with different substrates. Plant Soil 371:435–46.
Ollinger SV, Smith M-L. 2005. Net Primary Production and Canopy Nitrogen in a Temperate Forest Landscape: An Analysis Using Imaging Spectroscopy, Modeling and Field Data. Ecosystems 8:760–78.
Ollinger SV, Smith ML, Martin ME, Hallett RA, Goodale CL, Aber JD. 2002. Regional Variation in Foliar Chemistry and N Cycling Among Forests of Diverse History and Composition*. Ecology 83:339–55.
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I. 2007. Specific root length as an indicator of environmental change. Plant Biosystems—An International Journal Dealing with all Aspects of Plant Biology 141:426–42.
Ouimette A, Guo D, Hobbie E, Gu J. 2013. Insights into root growth, function, and mycorrhizal abundance from chemical and isotopic data across root orders. Plant Soil 367:313–26.
Ouimette AP, Ollinger SV, Richardson AD, Hollinger DY, Keenan TF, Lepine LC, Vadeboncoeur MA. 2018. Carbon fluxes and interannual drivers in a temperate forest ecosystem assessed through comparison of top-down and bottom-up approaches. Agricultural and Forest Meteorology.
Pardo LH, Templer PH, Goodale CL, Duke S, Groffman PM, Adams MB, Boeckx P, Boggs J, Campbell J, Colman B, Compton J, Emmett B, Gundersen P, Kjønaas J, Lovett G, Mack M, Magill A, Mbila M, Mitchell MJ, McGee G, McNulty S, Nadelhoffer K, Ollinger S, Ross D, Rueth H, Rustad L, Schaberg P, Schiff S, Schleppi P, Spoelstra J, Wessel W. 2006. Regional Assessment of N Saturation using Foliar and Root \varvec {\delta}^{\bf 15}{\bf N}. Biogeochemistry 80:143–71.
Park BB, Yanai RD, Fahey TJ, Bailey SW, Siccama TG, Shanley JB, Cleavitt NL. 2008. Fine Root Dynamics and Forest Production Across a Calcium Gradient in Northern Hardwood and Conifer Ecosystems. Ecosystems 11:325–41.
Phillips RP, Finzi AC, Bernhardt ES. 2011. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecology Letters 14:187–94.
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H. 2009. Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proceedings of the Royal Society of London B: Biological Sciences 276:4237–45.
Raich JW, Nadelhoffer KJ. 1989. Belowground Carbon Allocation in Forest Ecosystems: Global Trends. Ecology 70:1346–54.
Read DJ, Perez-Moreno J. 2003. Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytologist 157:475–92.
Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C. 1998. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Functional Ecology 12:327–38.
Richardson AD, Braswell BH, Hollinger DY, Burman P, Davidson EA, Evans RS, Flanagan LB, Munger JW, Savage K, Urbanski SP, Wofsy SC. 2006. Comparing simple respiration models for eddy flux and dynamic chamber data. Agricultural and Forest Meteorology 141:219–34.
Ryan MG, Hubbard RM, Pongracic S, Raison RJ, McMurtrie RE. 1996. Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol 16:333–43.
Smith M-L, Martin ME. 2001. A plot-based method for rapid estimation of forest canopy chemistry. Can J For Res 31:549–55.
Smith M-L, Ollinger SV, Martin ME, Aber JD, Hallett RA, Goodale CL. 2002. Direct Estimation of Aboveground Forest Productivity Through Hyperspectral Remote Sensing of Canopy Nitrogen. Ecological Applications 12:1286–302.
Stephens RB, Remick TJ, Ducey MJ, Rowe RJ. 2017. Drivers of truffle biomass, community composition, and richness among forest types in the northeastern US. Fungal Ecology 29:30–41.
Vicca S, Luyssaert S, Peñuelas J, Campioli M, Chapin FS, Ciais P, Heinemeyer A, Högberg P, Kutsch WL, Law BE, Malhi Y, Papale D, Piao SL, Reichstein M, Schulze ED, Janssens IA. 2012. Fertile forests produce biomass more efficiently. Ecology Letters 15:520–6.
Vogt KA, Grier CC, Meier CE, Edmonds RL. 1982. Mycorrhizal Role in Net Primary Priduction and Nutrient Cytcling in Abies Amabilis Ecosystems in Western Washington. Ecology 63:370–80.
Wallander H, Ekblad A, Godbold DL, Johnson D, Bahr A, Baldrian P, Björk RG, Kieliszewska-Rokicka B, Kjøller R, Kraigher H, Plassard C, Rudawska M. 2013. Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils—A review. Soil Biology and Biochemistry 57:1034–47.
Waring RH, Running SW. 2010. Forest Ecosystems: Analysis at Multiple Scales. Elsevier
Yin H, Wheeler E, Phillips RP. 2014. Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biology and Biochemistry 78:213–21.
Acknowledgements
Thoughtful reviews by Michael Ryan, Rebecca Sanders-DeMott, Adrien Finzi, Gary Lovett, and Steve Frolking significantly improved the quality of the manuscript. Research at the Bartlett Experimental Forest is supported by the USDA Forest Service’s Northern Research Station. We acknowledge funding support from the following grants: National Science Foundation awards #DEB-1114804, #1638688, and #0614266; Northeastern States Research Cooperative #12DG11242307065; Hubbard Brook Long Term Ecological Research program, NSF 1114804; NH EPSCoR Program NSF Research Infrastructure Improvement Award # EPS 1101245; NASA Carbon Cycle Science Awards #NNX08AG14G and #NNX14AJ18G; NASA Terrestrial Ecology Award #NNX11AB88G; USDA National Institute of Food and Agriculture McIntire-Stennis Project (1006881). Partial funding was provided by the New Hampshire Agricultural Experiment Station. We also acknowledge the staff at Bartlett Experimental Forest, in particular Chris Costello, and the invaluable assistance of numerous undergraduate students over the last 15 years.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest
Additional information
Author Contributions
APO, SVO, EAH, LCL and MAV designed the study; RBS and RJR provided truffle abundance data; RBS, RJR, APO, SVO and EAH provided soil and root δ15N data from the truffle plots; LCL and SJT led field efforts for the collection of aboveground production and belowground fine root production, respectively. APO wrote the manuscript, with all authors contributing significantly to revisions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ouimette, A.P., Ollinger, S.V., Lepine, L.C. et al. Accounting for Carbon Flux to Mycorrhizal Fungi May Resolve Discrepancies in Forest Carbon Budgets. Ecosystems 23, 715–729 (2020). https://doi.org/10.1007/s10021-019-00440-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00440-3