Abstract
Cyclic phenomena have been the focus of many studies in stressed conifer forests. In these systems, suppressed seedlings are released following the synchronous death of canopy trees. These cycles occur over hundreds of years, and thus studying them in the field is difficult, if not impossible in some cases. This difficulty highlights the advantages of vegetation modeling studies. We used the individual-based gap model, University of Virginia Forest Model Enhanced (UVAFME), to simulate forest dynamics over time at a high-elevation, subalpine forest (dominated by Engelmann spruce and subalpine fir) in southern Wyoming. Following model calibration, UVAFME was validated by running it up an elevation gradient to determine if it could simulate changes in species composition with elevation. UVAFME was then run exclusively at the high-elevation location for periods of 3000 years to simulate long-term forest dynamics at the site. It was found that without the intrusion of exogenous disturbances, the subalpine zone of the Rocky Mountains demonstrates cyclic phenomena, both at the plot scale and the landscape scale. By itself, Engelmann spruce demonstrates a natural periodicity of 300 years, whereas subalpine fir has a natural periodicity of 200 years. In the two-species forest, both species have a periodicity of 300 years. This output corresponds well with field data from similar high-elevation conifer sites. These results, along with other examples of cyclic phenomena in ecological systems, indicate that periodicities in ecosystems may be more common than previously thought, though they may be difficult to distinguish due to disturbances and the time- and space-scales at which they occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1935 paper in which A.G. Tansley used the word “ecosystem” for the first time in print, he also distinguished between autogenic succession, in which dynamic change is brought about by feedbacks among plants and their habitat, and allogenic succession, in which the changes are the result of external factors. Most modern ecologists, as did Tansley in 1935, see ecosystem dynamics arising from a mixture of autogenic and allogenic factors. In the dynamics of a system, the part of the system response that arises from interactions among internal components often features feedbacks that can produce periodic sinusoidal variation. These embedded natural periodicities can reveal which frequencies in the external drivers of the system might excite increases in oscillations or instability in the system.
In forests, disturbances, such as fire, wind, and insect outbreaks, are the exogenous factors that most obviously excite ecosystem dynamics. “Space for time substitution” procedures are often applied to document long-term forest dynamics, through the study of ecosystem responses to equivalent disturbances on arguably similar ecosystems. The century-scale dynamics of forests make direct observation of their responses to exogenous factors quite difficult. This is doubly true for the endogenous dynamics of forests and their expected internal periodicities, as these cycles and waves of periodic variation can occur over hundreds of years. Hence, in situ studies on cyclical phenomena are difficult, if not impossible, especially if their cyclic nature is not visually obvious or spatially coordinated. Reconstructions can be developed using dendrochronology or pollen records, but even these methods are limited by the spatial and temporal extent of the data (Bugmann 2001). These limitations on direct observation implicate ecological models as a tool to investigate cyclic phenomena that result from exogenous and endogenous factors, and provide insight into which factors drive the cyclic behavior.
Several studies have provided clues of strongly cyclical internal forest dynamics. Watt (1947) in his classic “pattern and process” paradigm viewed forests and other ecosystems as mosaics with small-scale cyclical dynamics at the scale of a large dominant plant. Cyclic patterns in forests, those with a spatial aspect and those with only a temporal aspect, are seen as evidence for this underlying cyclic nature (Shugart and Woodward 2011). Indeed, Watt produced several examples of cyclic patterns in shrubs and herbaceous plants in stressful conditions in the Cairngorms of Scotland. Cyclical patterns of growth–dieback–regeneration cycles have been observed in other forests, notably in the ‘ohi’a (Metrosideros polymorpha) forests of Hawaii (Boeher and others 2013), the Scalesia forests of the Galapagos Islands (Itow 1988), various New Zealand forests (Jane and Green 1983), as well as others. The general pattern that is observed is one of exogenous factors, such as drought, producing episodic collapses of forest stands depending on endogenous preconditions, usually for large numbers of older or senescent trees. This cycle of a similar cohort of trees becoming dominant and subsequently dying all at once continues, generally with species-specific frequencies (McGee 1984; Sprugel 1984; Shugart 1984).
There has been a long history of documenting and studying cyclic phenomena in stressed conifer forests (Sprugel 1984; Reiners and Lang 1979; Sprugel and Bormann 1981; Shugart 1984; Moloney 1986). Balsam fir (Abies balsamea) in the subalpine forests of the northeastern U.S. exhibits a temporal and spatial wave regeneration pattern in which suppressed seedlings are released following the synchronous death of canopy trees, forming spatially coordinated waves of dead and regenerating trees (Sprugel 1984; Moloney 1986). This synchrony has been attributed to windthrow damage and environmental stress of exposed, older trees (Reiners and Lang 1979). A similar pattern also occurs in the high-elevation conifer forests of Japan (Sato 1994; Sato and Iwasa 1993; Kohyama 1982). The objective of our study is to apply an individual-based forest gap model to investigate the presence of periodicities in the internal forest dynamics of a high-elevation conifer forest in the Rocky Mountains of the western US.
Individual-based gap models, which simulate the establishment, growth, and death of individual trees on patches of a landscape, have the capability to simulate and track detailed forest dynamics through time (Shugart 1998). These models simulate the annual diameter increment growth for individual trees on patches, or “gaps,” about the size of a dominant tree crown (Bugmann 2001; Shugart and Woodward 2011). This annual growth is generally based on climate and soil processes, light, various stressors, and tree size. Trees compete with one another through shading and appropriation of resources. Simulated trees die due to decreased growth, and new trees establish in their place based on site, climate, and light conditions (Shugart and Woodward 2011). Gap models are valuable tools for studying cyclic phenomena in forests because they simulate small-scale annual processes, such as tree diameter increment growth and competition, which can be aggregated to larger spatial scales (that is, multiple hectares) and for extended time scales (that is, hundreds of years of simulation). Thus, gap models can simulate emergent properties of forest ecosystems that arise from multiple interacting processes at different time- and space-scales.
In this study, the individual-based gap model, University of Virginia Forest Model Enhanced (UVAFME), is used to simulate forest dynamics over time at a high-elevation, subalpine forest in southern Wyoming. It has been shown that the subalpine zone in this area may exhibit some cyclic phenomena (Aplet and others 1988); however, the temporal extent of that study was limited by the age of the oldest tree on the stand. Using UVAFME, forest dynamics over thousands of years were simulated to explore cyclic behavior at both the plot and landscape scale at this subalpine site.
Methods
Study Site
The Glacier Lakes Ecosystem Experiments Site (GLEES) is located in the Snowy Range of the Rocky Mountains at 41°22′30″N and 106°15′30″W at elevations from 3200 to 3500 m. GLEES is in the Medicine Bow National Forest managed by the USDA Forest Service. Average annual precipitation at the site is about 100 cm (Musselman and others 1994), mean July temperature is 24°C, and mean January temperature is −9°C. The climate and site conditions are in general extremely harsh for tree growth, and the forest is strongly influenced by climate. Subalpine fir (Abies lasiocarpa) and Engelmann spruce (Picea engelmannii) dominate the site (Wooldridge and others 1996). Both species are characterized as very shade tolerant, but subalpine fir is slightly more tolerant than is Engelmann spruce (Burns and Honkala 1990; Alexander 1987).
Model Description
The University of Virginia Forest Model Enhanced (UVAFME) is an object-oriented extension of the individual-based gap model FAREAST (Yan and Shugart 2005). A detailed description of subroutines and parameters can be found in Yan and Shugart (2005) and Shuman and others (2014). As a gap model, it computes the annual growth, death, and establishment of each tree on independent patches, which together comprise a forested landscape. The annual output of each simulated patch resembles a sample area with a tally of the diameter and species of each tree on the plot. Several hundred such simulated patches are averaged to produce an expected mean biomass and species composition of a forested landscape through time.
The biomass and species composition of the simulated forest are affected by competition between individual trees for resources. Throughout the simulation, changes in species’ seedling banks and to individual tree processes (that is, growth, regeneration, and biomass accumulation) are functions of changes in the vertical light profile, temperature, moisture, and nutrients. Species-specific input parameters determine the annual optimal diameter increment of each simulated tree as a function of tree size. This optimal increment is then modified according to the environment (light, temperature, and resource availabilities interacting with species-specific tolerances). In the individual tree competition, different species have resource-specific advantages over others. Competition occurs both between conspecific individuals as well as between individuals of other species. The probability of a tree dying is based on growth-related stress, and new trees are planted based on resource availability and species-specific resource requirements. Soil conditions for each plot, such as soil water content, soil carbon, and plant available nitrogen, are then computed annually using a coupled 3 soil-layer water, carbon, and nitrogen submodel driven by climate, environmental conditions, and available nutrients.
Yan and Shugart tested the FAREAST model’s ability to simulate forest composition and zonation along an elevation gradient on Changbai Mountain in China, and subsequently tested its ability to simulate different forest types at 31 sites across eastern Russia (Yan and Shugart 2005). Forest composition and biomass output from both tests showed agreement with forest inventory data, implying the model’s ability to simulate forest compositional dynamics at both the local and regional scales. Additional model validation against 44 well-studied locations across all of Russia showed that results capture natural biomass accumulation rates without recalibration with appropriate responsiveness to local site and climate variability and demonstrate strong correlations to inventoried forest biomass (Shuman and others 2014).
UVAFME was calibrated to the GLEES site using data on species composition, climate, and site and soil parameters from the SNOTEL Network (NRCS 2014) and the US Forest Service. Daily precipitation and temperature conditions in UVAFME are derived from statistical distributions of mean monthly precipitation and temperature from 24 years of weather station data from the site for the period of 1989 to 2013 (NRCS 2014). Precipitation is also used to update soil water content on a daily basis. Species-specific parameter inputs for eleven major species found in the greater Rocky Mountain landscapes (Juniperus scopulorum, Pinus edulis, P. contorta, P. ponderosa, P. flexilis, Pseudotsuga menziesii, Populus tremuloides, P. angustifolia, Abies lasiocarpa, Picea engelmannii, and P. pungens; Burns and Honkala 1990; Peet 1981; Jose Negron, pers. comm.), such as maximum age, DBH, and height; stress tolerance levels; and temperature thresholds, are derived from Burns and Honkala (1990) (see Table 1). These inputs are used to determine species establishment at different locations. For each model simulation run, 200 independent, 500 m2 (0.05 ha) plots are simulated from bare ground to year 3000. The same soil and climate conditions influence each plot in a simulation run. The resultant Monte Carlo simulation produces a statistical sample of a larger forested landscape (Bormann and Likens 1979; Bugmann and others 1996).
Model Validation
UVAFME was validated against an elevation gradient on its ability to accurately simulate changes in species composition at different elevations. All eleven major species present in the Colorado and southern Wyoming Rocky Mountains were eligible for colonization at all elevations. This test used 200 independent plots in a Monte Carlo-style simulation for elevations from 1600 to 3600 m at 100-m intervals for 500 years. These simulations were initialized on “open plots” with no individual trees present at year 0 and the model assembled a forest and successional change of these plots. By year 500, the modeled forest community has stabilized, and reflects expected species composition for mature natural landscapes. No disturbances were used for these runs, as the internal dynamics (that is, the endogenous factors) of the system were the primary focus of this study. The composition of the forest community at year 500 for each elevation was compared to that observed on typical mountainsides in the Rocky Mountains (Marr 1961; Peet 1981).
Model Simulation of Subalpine Zone
UVAFME-simulated dynamics were inspected in more detail in the subalpine (3400 m) location, where subalpine fir (A. lasiocarpa) and Engelmann spruce (P. engelmannii) are expected to occur. To determine tree demography for both species and for the forest as a whole, the model was run under three different scenarios: (1) subalpine fir as the only available species; (2) Engelmann spruce only; and (3) with both subalpine fir and Engelmann spruce available. UVAFME was run for 200 independent plots in each scenario, starting with open plots, and the simulations were run for 3000 years. Again, disturbances were not used so that endogenous factors could be clearly studied.
Results
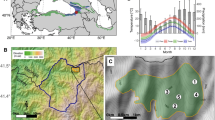
Figure 1 shows the validation results. The predicted zonation of species composition at year 500 corresponds well with that expected in the Rocky Mountains in this area (Peet 1981). A juniper-pinyon pine system develops at the lowest elevations, and gives way to a Douglas fir-ponderosa pine zone in middle elevations. At elevations above 2600 m, a subalpine zone dominated by subalpine fir and Engelmann spruce develops (Figure 1). From the set of eleven species, UVAFME closely resembles the smaller subsets of these species expected at each elevation zone with appropriate breakpoints in the simulated zonation. As in the Yan and Shugart (2005) validation in Chinese forests, these results demonstrate that UVAFME is capable of simulating species composition in response to the climate and soil conditions varying with elevation. It is expected that these results would vary if disturbances such as fire and windthrow were included in the simulation runs. Most notably, Pinus contorta, which regenerates after fire (Burns and Honkala 1990), would be expected to have higher biomass. These tests with fire and windthrow as well as quantitative comparisons of model output to forest inventory data are carried out in future studies.
The 3000-year simulations of the subalpine zone show cyclic phenomena that vary with the species mixture. For the first model scenario (exclusively subalpine fir), fir pulsates with a period of about 200 years (Figure 2A, B). This cyclic pattern occurs in both the stem count (Figure 2A) and biomass responses (Figure 2B). In the second model scenario, Engelmann spruce shows a periodicity of about 300 years (Figure 2C, D). With possible interspecies competition in the third model scenario, both Engelmann spruce and subalpine fir have a frequency of about 300 years (Figure 3). This cyclic pattern occurs at the plot level (Figure 3A, B) and at the landscape level (Figure 3C, D).
Stem count (A, C) and biomass (B, D) for a subalpine system (A. lasiocarpa and P. engelmannii) over 3000 years. Graphs A and B are for plot-level output of stem count (trees ha−1) and biomass (tC ha−1). Graphs C and D are stem count and biomass averaged over 200 plots (that is, landscape-level output).
To more clearly visualize the synchrony found in these cyclic patterns, the simulated biomass curves were detrended by subtracting the best-fit linear model through each species’ dynamics. These landscape-scale biomass curves clearly show that these species are almost exactly out of phase with each other; the peak of one species’ biomass occurs at the trough of the other’s (Figure 4).
Discussion
Several investigators have demonstrated evidence for long-term periodicities in forest ecosystems, despite the logistic challenges inherent making such direct observations. McGee (1984) used dendrochronological analyses to investigate a synchronized canopy dieback of century-old trees during a drought in a diverse uncut forest in East Tennessee to find large canopy trees demonstrating semi-synchronized mortality in two species. Mueller-Dombois (1986) reviews many examples of synchronized diebacks for a range of forest ecosystems at widespread locations. Green (1981) conducted a time series analysis on several 2000-year-old pollen cores from Everitt Lake, Nova Scotia, and found that there was a periodicity in the pollen data for many of the tree species, including fir, spruce, and pine, with periodicities ranging from 100 to 600 years. In a detailed reconstruction of Pinus sylvestris forest demographics, Zyabchenko (1982) found that P. sylvestris stands in the high-latitude forests of western Russia exhibit a cyclic pattern with a frequency of about 300 years. Space-for-time substitution studies on fir dynamics of Japan and the northeastern U.S. offer a clear visual example of cyclic phenomena that have a spatial component (that is, fir waves) (Sprugel 1984; Moloney 1986; Sato and Iwasa 1993). These observations suggest that the underlying periodicities in forest ecosystems, such as those described in our study, are more common that it may seem (Platt and Denman 1975). These endogenous periodicities are usually obscured at the human time scale by disturbances to the system (that is, hurricanes, logging, and so on).
Ecological modeling provides a unique opportunity for studying the endogenous properties of ecosystems, without the intrusion of exogenous factors. In individual-based gap models, periodic phenomena are emergent properties of local-scale forest dynamics. Periodicities in the dynamic responses of ecosystems are of interest because of the internal dynamics that they imply. This is particularly so when these dynamics arise as the consequences of internal interactions, or through autogenic succession. Fir (Abies spp.) waves are a rich example in forest ecosystems because they seem to be a chronosequence of the cyclical underlying patterns of change originally discussed by Watt (1947) for forests and other systems. Although Abies lasiocarpa in our model-based analysis is not known for wave regeneration, our study indicates that without the intrusion of disturbance, it could also produce a wave-like pattern. Though UVAFME is not spatially explicit, the fact that it produces cyclic phenomena in fir and spruce without spatial coordination and without regeneration by exogenous intrusions points to an underlying periodicity within the system. These internal periodicities, when organized by external environmental drivers as in Sprugel’s (1984) classic study, could produce the spatially coordinated fir waves seen in the northeastern US and Japan.
Based on the approximately 180° out-of-phase periodicity in the biomass peaks of spruce and fir in these simulations (Figure 3D), the cycles seen in the model resemble some sort of reciprocal replacement between the two tree species. There have been many recorded instances of reciprocal replacement in which the seedlings and saplings of one tree species are unable to regenerate under adults of the same species (Grubb 1977; Jones 1945; Schaeffer and Moreau 1958). This phenomenon has been attributed to a difference in an environmental factor experienced by the adult trees relative to that experienced by young trees. Light represents such a potential factor. When two shade-tolerant species co-dominate, they may repeatedly replace each other on the landscape.
For example, in American beech (Fagus grandifolia)–sugar maple (Acer saccharum) forests (Woods and Whittaker 1981; Woods 1979), both shade-tolerant species co-dominate the forest. In old growth stands of these species, beech and maple saplings tend to occur in areas that are closer to a canopy tree of the opposite species (Woods and Whittaker 1981). A study by Forcier (1975) found that the negative association between young trees and adult trees of the same species was a large driver of cyclical dynamics between yellow birch (Betula alleghaniensis), sugar maple, and beech in a New Hampshire forest. Although the dynamics seen in our study superficially resemble reciprocal replacement, with detailed inspection of the simulated stem count, reciprocal replacement does not seem to be the cause of the cyclic pattern in the subalpine zone. Both spruce and fir go through rapid regeneration at the same time on the individually simulated small plots, and not one after the other (Figure 3B, D), as would be expected for reciprocal replacement.
Cyclic behavior of forests has already been seen in other individual-based models (Emanuel and others 1978; Tharp 1978; Shugart 1984). For example, using a model-based analysis, Pastor and others (1987) showed cyclic dynamics between spruce and birch in boreal North America arising from nitrogen limitation interacting with forest demography. Emanuel and others (1978) found that the biomass output generated from the FORET model in an eastern U.S. hardwood forest had a strong cyclical component, with a frequency of about 200 years. The addition of a formerly dominant species (American chestnut, Castanea dentata) changed the frequency of the biomass cycle. A similar change in frequency is seen in the simulations of the subalpine zone of the Rocky Mountains in this study (Figures 2, 3). By itself, subalpine fir exhibits a strong periodicity of about 200 years (Figure 2), but with the addition of Engelmann spruce, which dominates subalpine fir, the periodicity of stem count and biomass changes to 300 years (Figure 3).
From the biomass and stem counts in three scenarios investigated, the cyclic pattern apparently results from the differences in the size and growth rate of fir and spruce. At the plot level (Figure 3A, B), with the initial forest establishment on a plot at year 0, subalpine fir outcompetes the slower-growing Engelmann spruce. Although subalpine fir grows very quickly initially, its rate of diameter increase drops rapidly at around year 100. In contrast, Engelmann spruce grows more slowly throughout its lifetime, and generally lives much longer than does subalpine fir (Burns and Honkala 1990; Veblen and others 1991). When the dominant age class of subalpine fir slows in growth around year 200 of the simulation, Engelmann spruce overtakes subalpine fir and becomes the dominant species (Figure 3A). As the older subalpine fir trees begin to die, neither new fir nor spruce can regenerate under the dense canopy of adult spruce trees, which typically have a higher maximum diameter and height than subalpine fir (Burns and Honkala 1990). These growth characteristics of spruce and fir are manifested in the model through the species-specific parameters AGEmax, DBHmax, and Hmax (Table 1). The diameter increment growth for each year (G, cm) is based on these parameters, and is calculated in part from the growth equation of Botkin and others (1972):
where DBHmax is the species-specific maximum diameter at breast height for the tree (cm), Hmax is the maximum height (cm), AGEmax is the maximum age (years), and a = ((1-1.37)/Hmax. Through this equation, the increment growth for each tree slows as it ages, according to its own species-specific parameters. These input parameters can be found in Table 1, and were derived chiefly from Burns and Honkala (1990).
Once Engelmann spruce becomes dominant, those few dominant trees are large enough to suppress all seedlings. Eventually at around year 300, the old canopy spruce trees begin to senesce and are increasingly susceptible to environmental stress. In this window, a series of unfortunate events in the form of multiple bad years kill the canopy spruce. Suppressed trees in the subcanopy and understory are released. Fir, due to its higher growth rate, is able to outperform the young spruce and the cycle repeats (see Figure 6 for a simplified drawing of this cycle).
The plot-level output from this study also corresponds with what has been seen in some field studies on forest demographics in stressed conifer systems (Zyabchenko 1982; Aplet and others 1988). Zyabchenko (1982) conducted an intensive field campaign on Pinus sylvestris stands in the high-latitude forests of western Russia featuring massive and detailed volumes of data collection from over 24 plots in order to reconstruct basal area, biovolume (m3 C ha−1), and average accumulation of biovolume, stems per hectare, and average DBH and height for a 650-year chronosequence. Through this rigorous investigation (in which over 1700 trees were cut down and over 1900 saplings were cored), it was found that P. sylvestris exhibits a cyclic canopy breakup and explosive increases in regeneration, with a periodicity of about 300 years (Figure 5A, B). These data closely resemble the output from UVAFME for the subalpine zone (Figure 5). This type of forest dynamics analysis from in situ data is only feasible using such comprehensive and exhaustive field methods. Ecological modeling allows us to examine these phenomena without such labor-intensive, time-consuming methods.
Internal wave dynamics in cold systems. (A) Biovolume (m3C ha−1) of Pinus sylvestris (redrawn from Zyabchenko 1982). The I, II, and III notations indicate cohorts of trees, that is, individual generations; (B) stem count (trees ha−1) of P. sylvestris in western Russia (redrawn from Zyabchenko 1982); (C) relative basal area from the third modeling scenario for fir (dashed line), spruce (solid line) from year 0 to year 900 alongside relative basal area data from Aplet and others (1988) for fir (open circle) and spruce (solid circle).
Aplet and others (1988) created a 600-year chronosequence of changes in age structure of an Engelmann spruce–subalpine fir forest in Colorado using field and dendrochronological data from five stands, and through this chronosequence, a cyclic spruce–fir basal area pattern emerged. They theorized that there were four phases of spruce–fir dynamics: colonization, in which both spruce and fir seedlings regenerate on the stand; spruce exclusion, in which spruce is initially inhibited by fir; spruce reinitiation, in which spruce outcompetes fir; and second generation spruce–fir forest, the final phase of the spruce–fir dynamics, in which the basal area of spruce and fir stabilizes. The UVAFME output from bare ground to year 500 for the third model scenario (the competitive scenario of spruce and fir) (Figure 3A) corresponds to the basal area pattern from Aplet and others (1988) (Figure 5C) and to the first three theorized phases. Although the fits between the two datasets are not perfect, the changes in the basal area trend match well. The study by Aplet and others (1988) did not have field data past year 575, so it is not certain how the respective basal areas of spruce and fir may have changed. The UVAFME output suggests that without disturbance, the periodic cycle of spruce and fir may continue into the future.
The results of this current study only use endogenous mortality due to tree stress or low growth. Disturbance by fire, wind, and insects are not included in these simulations. These types of disturbances are integral parts of the subalpine landscape; however, we were able to better visualize the potential underlying periodicities of the system with disturbances “turned off.” Without random disturbance, secondary succession starts at the same time for all 200 plots, which sets in motion the cyclical pattern of both species. This repeating cycle can be seen at both the plot scale (Figure 3A, B), and at the landscape scale (Figure 3C, D), indicating that most of the 200 plots in the model are fairly in sync. This is likely occurring due to the strong influence of climate on this site and the absence of disturbance in the model. The subalpine zone is literally the edge of these trees’ tolerance zones. On mountains that extend past 3600 meters or so, the trees turn stunted and hunch over into “Krummholz” forms, beaten down by icy wind and cold. With no disturbances in the model, these trees are so influenced by climate that they are synchronized when the combination of tree senescence and the occurrence of a run of stressful years kill most of the dominant old trees on most of the plots. One expects that under normal conditions in the field, disturbances like fire, windthrow, and insect outbreaks disrupt this endogenous pattern by “resetting” the internal cycle on different plots. This produces a landscape comprising plots that may be at a different stage at any one time, a quasi-equilibrium forest landscape mosaic (Bormann and Likens 1979; Shugart 1984).
This study has implications for an alternate subalpine landscape under an alternate climate regime. Most of the plot-scale cycle is relatively predictable. Once spruce and fir regenerate at the same time, spruce will eventually outcompete fir, suppress fir and spruce seedlings, and then eventually release both species through synchronous mortality. This self-perpetuating cycle creates the conditions (that is, simultaneous spruce and fir regeneration) for it to continue into the future. There is only a small “window of opportunity” in between spruce mortality and the spruce/fir regeneration for the pattern to go in a different direction (Shugart and others 1986). If, for example, fir were to establish in greater numbers than spruce, fir may successfully suppress and eventually overtake spruce as the dominant species on the stand. This small window of opportunity introduces the ability for changes in climate to drastically alter the dynamics of the subalpine zone (Figure 6).
In a study by Elliott (2011), it was found that while factors affecting successful regeneration were strong drivers of forest dynamics in the subalpine zone, climate has the ability to change these driving factors. With climate change, tree species may become more vulnerable to drought and warmer temperatures (Anderegg and others 2012), and Engelmann spruce seedlings are known to be generally intolerant to high temperatures (Seidel 1986). Additionally, increases in spruce beetle (Dendroctonus rufipennis) outbreaks may further reduce Engelmann spruce dominance at the plot scale (Berg and others 2006). The greater stem density of fir may then allow it to quickly overtake the temperature-intolerant, beetle-sensitive spruce (Seidel 1986). These changes in species dominance at the plot level may then scale up to changes in species dominance across the subalpine landscape.
Conclusions
Periodic phenomena in forest ecosystems have been variously studied using pollen records, dendrochronological reconstructions, space-for-time substitutions, and intensive and long-term forest sampling campaigns. To study these cyclic phenomena, which often have periodicities of hundreds of years, long-term datasets that are unmarred by disturbance events are a necessity. It is no small wonder that studies of this nature are few and far between. Using field methods alone, it would be nearly impossible to find more than a few forest stands older than about 500 years, especially in the western United States, where stand-replacing disturbance events are an integral component of the ecosystem. Ecological modeling provides a tool to study autogenic succession and the forest dynamics resulting from endogenous factors, without the need for long-term inventory or reconstruction data. With thousands-of-years model output, and the ability to “turn off” stand-replacing disturbances, the long-term, internal dynamics of forest ecosystems can be studied. We have shown, through the use of the individual-based gap model UVAFME, that the subalpine zone of the Rocky Mountains may contain internal cyclic phenomena, with a periodicity of about 300 years. Without disturbance, this cycle of fir initiation, eventual spruce dominance, spruce dieback, and spruce/fir regeneration is self-perpetuating, as long as the initial conditions of spruce/fir regeneration are present. If the initial conditions were to change, due to climate change or a shift in disturbance frequency, the cycle may go in a new, different direction. Shifts in patterns and processes at the plot (that is, less than 1 ha) scale have the ability to effect changes at the landscape and regional scales. It is clear that processes such as the cyclic phenomena described here are important, not just from a theoretical perspective, but also in terms of how the greater Rocky Mountain landscape may change in the context of regional shifts in climate and disturbance.
References
Alexander R. 1987. Ecology, silviculture, and management of the Engelmann spruce—subalpine fir type in the central and southern Rocky Mountains. USDA Forest Service, Agriculture Handbook No. 659, 155 pp.
Anderegg WRL, Kane JM, Anderegg LDL. 2012. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat Climate Change. doi:10.1038/nclimate1635.
Aplet GH, Laven RD, Smith FW. 1988. Patterns of community dynamics in Colorado Engelmann spruce-subalpine fir forests. Ecology 69:312–19.
Berg EE, Henry JD, Fastie CL, De Volder AD, Matsuoka SM. 2006. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: relationship to summer temperatures and regional differences in disturbance regimes. For Ecol Manage 227:219–32.
Boeher HJ, Wagner HH, Jacobi JD, Gerrish GC, Mueller-Domois D. 2013. Rebuilding after collapse: evidence for long-term cohort dynamics in the native Hawaiian rain forest. J Veg Sci 24:639–50.
Bormann FH, Likens GE. 1979. Pattern and process in a forested ecosystem. New York: Springer-Verlag. p 253p.
Botkin DB, Janak JF, Wallis JR. 1972. Some ecological consequences of a computer model of forest growth. J Ecol 60:849–72.
Bugmann H. 2001. A review of forest gap models. Clim Change 51:259–305.
Bugmann H, Fishchlin A, Kienast F. 1996. Model convergence and state variable update in forest gap models. Ecol Model 89:197–208.
Burns RM, Honkala BH. 1990. Silvics of North America: 1. Conifers; 2. Hardwoods. Agricultural handbook 654. U.S. Department of Agriculture, Forest Service Washington D.C. vol. 2, 877 pp.
Elliott GP. 2011. Influences of 20th-century warming at the upper tree line contingent on local-scale interactions: evidence from a latitudinal gradient in the Rocky Mountains, USA. Glob Ecol Biogeogr 20:46–57.
Emanuel WR, West DC, Shugart HH. 1978. Spectral analysis of forest model time series. Ecol Model 4:313–26.
Forcier LK. 1975. Reproductive strategies and the co-occurrence of climax tree species. Science 189:808–10.
Green DG. 1981. Time series and postglacial forest ecology. Quatern Res 15:265–77.
Grubb PJ. 1977. The maintenance of species richness in plant communities. The importance of the regeneration niche. Biol Rev 52:107–45.
Itow S. 1988. Population structure, stand-level dieback and recovery of Scalesia pedunculata forest in the Galapagos Islands. Ecol Res 3:333–9.
Jane GT, Green TGA. 1983. Vegetation mortality in the Kaimai Ranges, North Island, New Zealand. Pac Sci 37:385–9.
Jones EW. 1945. The structure and reproduction of the virgin forest of the North Temperate zone. New Phytol 44:130–48.
Kohyama T. 1982. Studies on the Abies population of Mt. Shimagare II. Reproductive and life history traits. Bot Magazine Tokyo 95:167–81.
Marr JW. 1961. Ecosystems of the east slope of the Front Range in Colorado. Univ. Colorado Studies in Biology 8. University of Colorado, Boulder. 134 pp.
McGee CE. 1984. Heavy mortality and succession in a virgin mixed mesophytic forest. Research paper SO-209. New Orleans, LA: U.S. Dept. of Agriculture, Forest Service, Southern Forest Experiment Station.
Moloney KA. 1986. Wave and nonwave regeneration processes in a subalpine Abies balsamea forest. Can J Bot 64:341–9.
Musselman RC, Fox DG, Schoettle AW, Regan CM. 1994. The Glacier lakes ecosystem experiments site. U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station 94 pp.
Mueller-Dombois D. 1986. Perspectives for an etiology of stand-level dieback. Annu Rev Ecol Syst 17:221–43.
NRCS. 2014. Natural resources conservation service. National Water and Climate Center: SNOTEL Site Brooklyn Lake. http://www.wcc.nrcs.usda.gov/nwcc/site?sitenum=367.
Pastor J, Gardner RH, Dale VH, Post WM. 1987. Successional changes in nitrogen availability as a potential factor contributing to spruce declines in boreal North America. Can J For Res 17:1394–400.
Peet RK. 1981. Forest vegetation of the Colorado Front Range: composition and dynamics. Vegetatio 45:3–75.
Platt T, Denman KL. 1975. Spectral analysis in ecology. Annu Rev Ecol Syst 6:189–210.
Reiners WA, Lang GE. 1979. Vegetational patterns and processes in the balsam fir zone, White Mountains, New Hampshire. Ecology 60:403–17.
Sato T. 1994. Stand structure and dynamics of wave-type Abies sachalinensis coastal forest. Ecol Res 9:77–84.
Sato K, Iwasa Y. 1993. Modeling of wave regeneration in subalpine forests: population dynamics with spatial structure. Ecology 74:1538–50.
Schaeffer R, Moreau R. 1958. L’alternance des essences. Society For. France Compte Bull. 29:1–12, 76–84, 277–298.
Seidel KW. 1986. Tolerance of seedlings of ponderosa pine, Douglas fir, grand fir, and Engelmann spruce for high temperatures. Northwest Sci 60:7p.
Shugart HH, Woodward FI. 2011. Global change and the terrestrial biosphere. West Sussex (NJ): Wiley-Blackwell. p 242p.
Shugart HH, Antonovsky, MJa, Jarvis PG, Sanford AP. 1986. CO2, climatic change, and forest ecosystems: assessing the response of global forests to the direct effects of increasing CO2 and climatic change. In: Bolin B, Doos BR, Jager J, Warrick RA, Eds. The greenhouse effect, climate change and ecosystems (SCOPE 29). New York: John Wiley. pp 475–521.
Shugart HH. 1984. A theory of forest dynamics: the ecological implications of forest succession models. New York: Springer Verlag (Reprinted: 2003, New Jersey: The Blackburn Press). 278 pp.
Shugart HH. 1998. Terrestrial ecosystems in changing environments. Cambridge (UK): Cambridge University Press. p 537p.
Shuman JK, Shugart HH, Krankina ON. 2014. Testing individual-based models of forest dynamics: issues and an example for the boreal forests of Russia. Ecol Model 293:102–10.
Sprugel DG. 1984. Density, biomass, productivity, and nutrient-cycling changes during stand development in wave-regenerated balsam fir forests. Ecol Monogr 54:165–86.
Sprugel DG, Bormann FH. 1981. Natural disturbance and the steady state in high-altitude balsam fir forests. Science 211:390–3.
Tansley AG. 1935. The use and abuse of vegetational concepts and terms. Ecology 16:284–307.
Tharp ML. 1978. Modeling major perturbations on a forest ecosystem. University of Tennessee, Knoxville. M.S. Thesis.
Veblen TT, Hadley KS, Reid MS, Rebertus AJ. 1991. The response of subalpine forests to spruce beetle outbreak in Colorado. Ecology 72:213–31.
Watt AS. 1947. Pattern and process in the plant community. J Ecol 35:1–22.
Wooldridge GL, Musselman RC, Sommerfield RA, Fox DG, Connell BH. 1996. Mean wind patterns and snow depths in an alpine-subalpine ecosystem as measured by damage to coniferous trees. J Appl Ecol 33:100–8.
Woods KD, Whittaker RH. 1981. Canopy-understory interaction and the internal dynamics of mature hardwood and hemlock-hardwood forests. West DC, Shugart HH, Botkin DB, editors. Forest succession: concepts and application. New York: Springer-Verlag. pp 305–323.
Woods KD. 1979. Reciprocal replacement and the maintenance of codominance in a beech-maple forests. Oikos 33:31–9.
Yan X, Shugart HH. 2005. FAREAST: a forest gap model to simulate dynamics and patterns of eastern Eurasian forests. J Biogeogr 32:1641–58.
Zyabchenko SS. 1982. Age dynamics of scotch pine forests in the European North. Lesovedenie 2:3–10.
Acknowledgements
This work was funded by the VA Space Grant Consortium Graduate Fellowship (VSGC FY 14-15 A Foster, project title: “Understanding spruce beetle outbreak dynamics and their response to climate change through remote sensing and ecological modeling”), by a Grant from the National Fish and Wildlife Foundation (Grant Number: 0106.12.032847), and through the UVA Programs of Distinction. We thank N. Vercruysse for helpful comments on this manuscript, Ksenia Brazhnik for help with Russian translations, Robert Smith for figure preparation, Jose Negron for advice about the subalpine system, and Katherine Holcomb and the UVACSE team for coding expertise and help with model development. We also thank two anonymous reviewers and John Pastor for their very useful comments during the editing and revising of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
A.C.F. conceived of, designed, and executed the experiment, conducted background research, and wrote the manuscript. H.H.S. helped conceive of the idea, conducted background research, and helped write the manuscript. J.K.S. helped with model calibration and writing the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Foster, A.C., Shugart, H.H. & Shuman, J.K. Model-based Evidence for Cyclic Phenomena in a High-Elevation, Two-Species Forest. Ecosystems 19, 437–449 (2016). https://doi.org/10.1007/s10021-015-9945-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-015-9945-y