Abstract
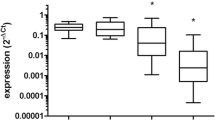
MicroRNAs (miRNAs) are small noncoding RNAs that take part in diverse biological processes by suppressing target gene expression. Elevated expression of miR-21 has been reported in many types of human cancers. Radiotherapy is a standard adjuvant treatment for patients with glioblastoma. However, the resistance of glioblastoma cells to radiation limits the success of this treatment. In this study, we found that miR-21 expression was upregulated in response to ionizing radiation (IR) in U251 cells, which suggested that miR-21 could be involved in the response of U251 cells to radiation. We showed that a miR-21 inhibitor enhanced IR-induced glioblastoma cell growth arrest and increased the level of apoptosis, which was probably caused by abrogation of the G2-M arrest induced by IR. Further research demonstrated that the miR-21 inhibitor induced the upregulation of Cdc25A. Taken together, these findings suggest that miR-21 inhibitor can increase IR-induced growth arrest and apoptosis in U251 glioblastoma cells, at least in part by abrogating G2-M arrest, and that Cdc25A is a potential target of miR-21.




Similar content being viewed by others
References
Sathornsumetee S, Rich JN (2006) New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther 6:1087–1104
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Cho WC (2007) OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer 6:60
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033
Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K (2007) MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res 67:8994–9000
Papagiannakopoulos T, Shapiro A, Kosik KS (2008) MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res 68(19):8164–8172
Bucher N, Britten CD (2008) G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 98:523–528
Boutros R, Dozier C, Ducommun B (2006) The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol 18:185–191
Ray D, Kiyokawa H (2008) CDC25A phosphatase: a rate-limiting oncogene that determines genomic stability. Cancer Res 68:1251–1253
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, Wang B, Wang G, Jia Z, Pu P, Zhang W, Kang C (2010) Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest 90:144–155
Kobayashi T, Masumoto J, Tada T, Nomiyama T, Hongo K, Nakayama J (2007) Prognostic significance of the immunohistochemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin Cancer Res 13:3868–3874
Bayascas JR, Yuste VJ, Benito E, Garcia-Fernandez J, Comella JX (2002) Isolation of AmphiCASP-3/7, an ancestral caspase from amphioxus (Branchiostoma floridae). Evolutionary considerations for vertebrate caspases. Cell Death Differ 9:1078–1089
Tsuboi K, Moritake T, Tsuchida Y, Tokuuye K, Matsumura A, Ando K (2007) Cell cycle checkpoint and apoptosis induction in glioblastoma cells and fibroblasts irradiated with carbon beam. J Radiat Res (Tokyo) 48:317–325
Mailand N, Podtelejnikov AV, Groth A, Mann M, Bartek J, Lukas J (2002) Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J 21:5911–5920
Chang JE, Khuntia D, Robins HI, Mehta MP (2007) Radiotherapy and radiosensitizers in the treatment of glioblastoma multiforme. Clin Adv Hematol Oncol 5:894–902, 907–915
Maes OC, An J, Sarojini H, Wu H, Wang E (2008) Changes in MicroRNA expression patterns in human fibroblasts after low-LET radiation. J Cell Biochem 105:824–834
Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ (2007) MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res 67:11111–11116
Ishii H, Saito T (2006) Radiation-induced response of micro RNA expression in murine embryonic stem cells. Med Chem 2:555–563
Wagner-Ecker M, Schwager C, Wirkner U, Abdollahi A, Huber PE (2010) MicroRNA expression after ionizing radiation in human endothelial cells. Radiat Oncol 5:25
Pothof J, Verkaik NS, van IW, Wiemer EA, Ta VT, van der Horst GT, Jaspers NG, van Gent DC, Hoeijmakers JH, Persengiev SP (2009) MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J 28:2090–2099
Zhu Y, Yu X, Fu H, Wang H, Wang P, Zheng X, Wang Y (2010) MicroRNA-21 is involved in ionizing radiation-promoted liver carcinogenesis. Int J Clin Exp Med 3:211–222
Hirose Y, Berger MS, Pieper RO (2001) Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res 61:5843–5849
Tentori L, Portarena I, Torino F, Scerrati M, Navarra P, Graziani G (2002) Poly(ADP-ribose) polymerase inhibitor increases growth inhibition and reduces G(2)/M cell accumulation induced by temozolomide in malignant glioma cells. Glia 40:44–54
Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, Arumugarajah S, Hylander-Gans L, Morosini D, Simeone DM, Canman CE, Normolle DP, Zabludoff SD, Maybaum J, Lawrence TS (2010) Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res 70:4972–4981
Frosina G (2009) DNA repair and resistance of gliomas to chemotherapy and radiotherapy. Mol Cancer Res 7:989–999
Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ Jr, Lazo JS, Wang Z, Zhang L, Yu J (2009) microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res 69:8157–8165
Acknowledgments
The authors thank Dr. Runze Xun and Dr. Jingshan Lu for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Li and S. Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10014_2011_37_MOESM1_ESM.tif
Additional Fig. 1. When DNA is damaged by radiation (IR), CHK1 is activated, and CHK1 then targets cell division cycle 25A (Cdc25A) to ubiquitin-mediated degradation. The CHK1-dependent degradation of Cdc25A is a major p53-independent mechanism of either cell-cycle arrest or a delay in the G2-M phase. Eventually, DNA damage results in cell-cycle arrest at G2-M. The G2 checkpoint has a protective function; therefore, the fate of the injured cells is determined by the extent of DNA damage and the duration of G2-M. The restored cells thereafter survive, whereas the unrestored cells undergo apoptosis (TIFF 38590 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Zhao, S., Zhen, Y. et al. A miR-21 inhibitor enhances apoptosis and reduces G2-M accumulation induced by ionizing radiation in human glioblastoma U251 cells. Brain Tumor Pathol 28, 209–214 (2011). https://doi.org/10.1007/s10014-011-0037-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-011-0037-1