Abstract
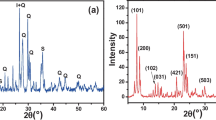
Nanozeolites A and X were synthesized via hydrothermal method using fine fraction of beneficiated clay. The beneficiation was achieved by collecting fine fraction of clay from high-quartz content Cameroon kaolin using Stoke’s law principle. This treatment has led to a significant increase of kaolinite content of the clay (80%) as well as its crystallinity whereas the associated impurity decreased. The synthesized zeolites and its clay precursor were characterized by X-ray fluorescence, X-ray diffraction, scanning electron microscopy, and infrared spectroscopy as well as argon adsorption–desorption isotherm. The results have shown that the cubic crystal nanozeolite A and an octahedral-like crystal of nanozeolite X with average crystal size of 63 nm and 82 nm and surface area of 58 m2/g and 860 m2/g were obtained after shorter crystallization time: 2 and 12 h, respectively. The obtained nanozeolites were used to modify carbon paste electrode for the sensitive electrocatalytic detection of acetaminophen and epinine using LSV compared with the unmodified electrode after the study of the electrochemical behavior of both analytes at zeolites type A and X-modified electrodes. The hypothesis of extrazeolite mechanisms was proposed for the electrochemical pathway sensing of AC and EP at zeolite-modified electrodes. The oxidation peak current for both modified electrodes showed linear dependence on concentration range 0.5–70 μM with detection limit of 0.2 μM and 0.3 μM for AC and 0.08 μM and 0.2 μM for EP at ZX-CPE and ZA-CPE, respectively.






Similar content being viewed by others
References
Karimi-Maleh H, Sheikhshoaie M, Sheikhshoaie I, Ranjbar M, Alizadeh J, Maxakato NW et al (2019) A novel electrochemical epinine sensor using amplified CuO nanoparticles and an n-hexyl-3-methylimidazolium hexafluorophosphate electrode. New J Chem 43(5):2362–2367. https://doi.org/10.1039/C8NJ05581E
Patel F (1992) The fatal paracetamol dosage–how low can you go? Med Sci Law 32(4):303–310. https://doi.org/10.1177/002580249203200404
Vilchez J, Blanc R, Avidad R, Navalón A (1995) Spectrofluorimetric determination of paracetamol in pharmaceuticals and biological fluids. J Pharm Biomed Anal 13(9):1119–1125. https://doi.org/10.1016/0731-7085(95)01537-U
Wei S, Song G, Lin J-M (2005) Separation and determination of norepinephrine, epinephrine and isoprinaline enantiomers by capillary electrophoresis in pharmaceutical formulation and human serum. J Chromatogr A 1098(1):166–171. https://doi.org/10.1016/j.chroma.2005.08.038
Lecoeur M, Rabenirina G, Schifano N, Odou P, Ethgen S, Lebuffe G et al (2019) Determination of acetaminophen and its main metabolites in urine by capillary electrophoresis hyphenated to mass spectrometry. Talanta 205:120108. https://doi.org/10.1016/j.talanta.2019.07.003
Boomsma F, Alberts G, van der Hoorn FAJ, Man in't Veld AJ, Schalekamp MADH (1992) Simultaneous determination of free catecholamines and epinine and estimation of total epinine and dopamine in plasma and urine by high-performance liquid chromatography with fluorimetric detection. J Chromatogr B Biomed Sci Appl 574(1):109–17. https://doi.org/10.1016/0378-4347(92)80104-X
He H, Stein CM, Christman B, Wood AJJ (1997) Determination of catecholamines in sheep plasma by high-performance liquid chromatography with electrochemical detection: comparison of deoxyepinephrine and 3,4-dihydroxybenzylamine as internal standard. J Chromatogr B Biomed Sci Appl 701(1):115–119. https://doi.org/10.1016/S0378-4347(97)00343-5
Kemmegne-Mbouguen JC, Ngameni E (2017) Simultaneous quantification of dopamine, acetaminophen and tyrosine at carbon paste electrodes modified with porphyrin and clay. Anal Methods 9(28):4157–4166. https://doi.org/10.1039/C7AY01173C
Atta NF, El-Kady MF, Galal A (2010) Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor. Anal Biochem 400(1):78–88. https://doi.org/10.1016/j.ab.2010.01.001
Vidyadharan AK, Jayan D, Mary Nancy TE (2014) Ni0.1Co0.9Fe2O4-based electrochemical sensor for the detection of paracetamol. J Solid State Electrochem 18(9):2513–9. https://doi.org/10.1007/s10008-014-2476-1
Raoof JB, Chekin F, Ojani R, Barari S, Anbia M, Mandegarzad S (2012) Synthesis and characterization of ordered mesoporous carbon as electrocatalyst for simultaneous determination of epinephrine and acetaminophen. J Solid State Electrochem 16(12):3753–3760. https://doi.org/10.1007/s10008-012-1807-3
Yang G, Wang L, Jia J, Zhou D, Li D (2012) Chemically modified glassy carbon electrode for electrochemical sensing paracetamol in acidic solution. J Solid State Electrochem 16(9):2967–2977. https://doi.org/10.1007/s10008-012-1713-8
Li S-J, Deng D-H, Pang H, Liu L, Xing Y, Liu S-R (2012) Preparation of electrochemically reduced graphene oxide-modified electrode and its application for determination of p-aminophenol. J Solid State Electrochem 16(9):2883–9. https://doi.org/10.1007/s10008-012-1720-9
Yang G, Yu L, Jia J, Zhao Z (2012) 4-Aminobenzoic acid covalently modified glassy carbon electrode for sensing paracetamol at different temperatures. J Solid State Electrochem 16(4):1363–1368. https://doi.org/10.1007/s10008-011-1529-y
Walcarius A (2008) Electroanalytical applications of microporous zeolites and mesoporous (organo)silicas: recent trends. Electroanalysis 20(7):711–738. https://doi.org/10.1002/elan.200704144
Ríos CA, Williams CD, Fullen MA (2009) Nucleation and growth history of zeolite LTA synthesized from kaolinite by two different methods. Appl Clay Sci 42(3):446–454. https://doi.org/10.1016/j.clay.2008.05.006
Rolison DR (1990) Zeolite-modified electrodes and electrode-modified zeolites. Chem Rev 90(5):867–878. https://doi.org/10.1021/cr00103a011
Mintova S, Gilson JP, Valtchev V (2013) Advances in nanosized zeolites. Nanoscale 5(15):6693–6703. https://doi.org/10.1039/c3nr01629c
Mintova S, Jaber M, Valtchev V (2015) Nanosized microporous crystals: emerging applications. Chem Soc Rev 44(20):7207–7233. https://doi.org/10.1039/C5CS00210A
Schoeman BJ, Sterte J, Otterstedt JE (1994) Colloidal zeolite suspensions. Zeolites 14(2):110–116. https://doi.org/10.1016/0144-2449(94)90004-3
Kannangara I, Jayawardhana Y, Munasinghe E, Rajapakse A, Bandara A, Weerasooriya R et al (2020) Synthesis and characterization of nano zeolite-A with aid of sodium dodecyl sulfate (SDS) as particle size-controlling agent. Colloids Surf A 589:124427. https://doi.org/10.1016/j.colsurfa.2020.124427
Boycheva S, Marinov I, Miteva S, Zgureva D (2020) Conversion of coal fly ash into nanozeolite Na-X by applying ultrasound assisted hydrothermal and fusion-hydrothermal alkaline activation. Sustain Chem Pharm 15100217-S2352554119301706:100217. https://doi.org/10.1016/j.scp.2020.100217
Sari ZGLV, Younesi H, Kazemian H (2015) Synthesis of nanosized ZSM-5 zeolite using extracted silica from rice husk without adding any alumina source. Appl Nanosci 5(6):737–745. https://doi.org/10.1007/s13204-014-0370-x
Sivalingam S, Sen S (2019) Valorization of coal fly ash into nanozeolite by sonication-assisted hydrothermal method. J Environ Manage 235145–151. S0301479719300428. https://doi.org/10.1016/j.jenvman.2019.01.042
Wang P, Sun Q, Zhang Y, Cao J (2020) Effective removal of methane using nano-sized zeolite 4A synthesized from kaolin. Inorg Chem Commun 111107639–107639. S138770031931010X. https://doi.org/10.1016/j.inoche.2019.107639
Ngoc DT, Pham TH, Nguyen KD (2013) Synthesis characterization and application of nanozeolite NaX from Vietnamese kaolin. Adv Nat Sci: Nanosci and Nanotechnol 4(4):045018. https://doi.org/10.1088/2043-6262/4/4/045018
Otieno SO, Kengara FO, Kemmegne-Mbouguen JC, Langmi HW, Kowenje CB, Mokaya R (2019) The effects of metakaolinization and fused-metakaolinization on zeolites synthesized from quartz rich natural clays. Microporous Mesoporous Mater 290:109668. S1387181119305256. https://doi.org/10.1016/j.micromeso.2019.109668
Lagaly G (2006) Chapter 5 Colloid Clay Science. In: Bergaya F, Theng BKG, Lagaly G (eds) Developments in Clay Science, vol 1. Elsevier, pp 141-245. https://doi.org/10.1016/S1572-4352(05)01005-6
Chandrasekhar S, Pramada PN (1999) J Porous Mater 6(4)283–297. https://doi.org/10.1023/A:1009632606671
Saikia BJ (2010) Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J Mod Phys 01:206
Alkan M, Hopa Ç, Yilmaz Z, Güler H (2005) The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater 86(1-3):176–184. S138718110500291X. https://doi.org/10.1016/j.micromeso.2005.07.008
Treacy MM, Higgins JB (2007) Collection of simulated XRD powder patterns for zeolites (fifth edition). Amsterdam: Elsevier Science B. V. p 10-6
Zhou Z, Jin G, Liu H, Wu J, Mei J (2014) Crystallization mechanism of zeolite A from coal kaolin using a two-step method. Appl Clay Sci 97-98:110–114. S0169131714001677. https://doi.org/10.1016/j.clay.2014.05.015
Ghasemi Z, Younesi H (2012) Preparation of Free-Template Nanometer-Sized Na–A and –X Zeolites From Rice Husk Ash. Waste Biomass Valorization 3(1):61–74. https://doi.org/10.1007/s12649-011-9084-4
Reinoso D, Adrover M, Pedernera M (2018) Green synthesis of nanocrystalline faujasite zeolite. Ultrason Sonochem 42:303–309. S1350417717305503. https://doi.org/10.1016/j.ultsonch.2017.11.034
Yamada H, Yokoyama S, Watanabe Y, Uno H, Tamura K (2005) Micro-cubic glass from pseudomorphism after thermal treatment of ammonium-exchanged zeolite A. Sci Technol Adv Mater 6(3-4):394–398. https://doi.org/10.1016/j.stam.2005.03.011
Loiola AR, Andrade JC, Sasaki JM, Da Silva LR (2012) Structural analysis of zeolite NaA synthesized by a cost-effective hydrothermal method using kaolin and its use as water softener. J Colloid Interface Sci 367(1):34–39. S0021979710013044. https://doi.org/10.1016/j.jcis.2010.11.026
Mozgawa W, Jastrzębski W, Handke M (2005) Vibrational spectra of D4R and D6R structural units. J Mol Struct 744-747:663–670. S0022286005000542. https://doi.org/10.1016/j.molstruc.2004.12.051
Flanigen EM, Khatami H, Szymanski HA (1974) Infrared Structural Studies of Zeolite Frameworks. In: Molecular Sieve Zeolites-I 101. Adv Clin Chem 101. American Chemical Society 201–229
Srilai S, Tanwongwal W, Onpecth K, Wongkitikun T, Panomsuwan G, Fuji M, Eiad-Ua A (2019) Influence of Crystallization Time for Synthesis of Zeolite A and Zeolite X from Natural Kaolin. Key Eng Mater 824:231–235. https://doi.org/10.4028/www.scientific.net/KEM.824.231
Sabatino D, Sabatino BD, Gimeno D, Pace C (2011) Synthesis and characterization of Na-X Na-A and Na-P zeolites and hydroxysodalite from metakaolinite. Clay Miner 46(3):339–354. https://doi.org/10.1180/claymin.2011.046.3.339
Mohiuddin E, Isa YM, Mdleleni MM, Sincadu N, Key D, Tshabalala T (2016) Synthesis of ZSM-5 from impure and beneficiated Grahamstown kaolin: Effect of kaolinite content crystallisation temperatures and time. Appl Clay Sci 119:213–221. S0169131715301368. https://doi.org/10.1016/j.clay.2015.10.008
Nematollahi D, Shayani-Jam H, Alimoradi M, Niroomand S (2009) Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis hydroxylation and dimerization processes. Electrochimica Acta 54(28):7407–7415. S001346860901024X. https://doi.org/10.1016/j.electacta.2009.07.077
Giesecke J (1976) The structure of the catecholamines. V. The crystal and molecular structure of epinine hydrobromide. Acta Crystallographica Section B Struct Cryst Cryst Chem 32(8):2337–2340. https://doi.org/10.1107/S0567740876007711
Huang J, Liu Y, Hou H, You T (2008) Simultaneous electrochemical determination of dopamine uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens Bioelectron 24(4):632–637. S0956566308002704. https://doi.org/10.1016/j.bios.2008.06.011
Majewska UE, Chmurski K, Biesiada K, Olszyna AR, Bilewicz R (2006) Dopamine Oxidation at Per(6-deoxy-6-thio)-α-Cyclodextrin Monolayer Modified Gold Electrodes. Electroanalysis 18(15):1463–1470. https://doi.org/10.1002/elan.200603556
Baker MD, Senaratne C, Zhang J (1992) Effects of supporting electrolyte and zeolite co-cations on the electrochemical response of zeolite-modified electrodes. J Chem Soc Faraday Trans 88(21):3187. https://doi.org/10.1039/ft9928803187
Shaw BR, Creasy KE, Lanczycki CJ, Sargeant JA, Tirhado M Voltammetric Response of Zeolite‐Modified Electrodes. J Electrochem Soc 135(4):869–876. https://doi.org/10.1149/1.2095814
Galhetas M, Mestre AS, Pinto ML, Gulyurtlu I, Lopes H, Carvalho AP (2014) Carbon-based materials prepared from pine gasification residues for acetaminophen adsorption. J Chem Eng 240:344–351. S1385894713015441. https://doi.org/10.1016/j.cej.2013.11.067
Walcarius A, Barbaise T, Bessiere J (1997) Factors affecting the analytical applications of zeolite-modified electrodes preconcentration of electroactive species. Analytica Chimica Acta 340(1-3):61–76. S0003267096005399. https://doi.org/10.1016/S0003-2670(96)00539-9
Walcarius A (1996) Zeolite-modified electrodes: Analytical applications and prospects. Electroanalysis 8(11):971–986. https://doi.org/10.1002/elan.1140081102
Walcarius A, Ganesan V, Larlus O, Valtchev V (2004) Low Temperature Synthesis of Zeolite Films on Glassy Carbon: Towards Designing Molecularly Selective Electrochemical Devices. Electroanalysis 16(18):1550–1554. https://doi.org/10.1002/elan.200302991
Gemborys HA, Shaw BR (1986) Electrochemical behavior of methyl viologen in zeolite particle films. J Electroanal Chem Interfacial Electrochem 208(1):95–107. https://doi.org/10.1016/0022-0728(86)90298-6
Wang J, Walcarius A (1996) Zeolite-modified carbon paste electrode for selective monitoring of dopamine. J Electroanal Chem 407(1-2):183–187. https://doi.org/10.1016/0022-0728(95)04488-4
Doménech-Carbó A (2015) Theoretical scenarios for the electrochemistry of porous silicate-based materials: an overview. J Solid State Electrochem 19(7):1887–1903. https://doi.org/10.1007/s10008-014-2690-x
Doménech A, García H, Alvaro M, Carbonell E (2003) Study of Redox Processes in Zeolite Y-Associated 246-Triphenylthiopyrylium Ion by Square Wave Voltammetry. J Phys Chem B 107(13):3040–3050. https://doi.org/10.1021/jp0223657
Li Z, Wang CM, Persaud L, Mallouk TE (1988) Electrochemistry of metalloporphyrins and viologens at zeolite Y-modified electrodes: evidence for electron trapping by monomolecular porphyrin layers. J Phys Chem 92(9):2592–2597. https://doi.org/10.1021/j100320a039
Kaur B, Srivastava R (2014) Selective Nanomolar Electrochemical Determination of Environmental Contaminants Dihydroxybenzene Isomers Found in Water Bodies Using Nanocrystalline Zeolite Modified Carbon Paste Electrodes. Electroanalysis 26(8):1739–1750. https://doi.org/10.1002/elan.201400171
Ding YP, Liu WL, Wu QS, Wang XG (2005) Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. J Electroanal Chem 575(2):275–280. S0022072804005066. https://doi.org/10.1016/j.jelechem.2004.09.020
Uzun D, Tabanlıgil Calam T (2022) Electrochemical Behavior and Ultrasensitive, Simple and Effective Voltammetric Determination of Acetaminophen Using Modified Glassy Carbon Electrode Based on 4-Hydroxyquinoline-3- Carboxylic Acid. Electroanalysis 34:1–12. https://doi.org/10.1002/elan.202200182
Sun L, Yang M, Guo H, Zhang T, Wu N, Wang M, Yang F, Zhang J, Yang W (2022) COOH-MWCNT connected COF and chemical activated CTF as a novel electrochemical sensing platform for simultaneous detection of acetaminophen and p-aminophenol. Colloids Surf A: Physicochem. Eng Aspects 647:129092. https://doi.org/10.1016/j.colsurfa.2022.129092
Wang P, Yuan X, Cui Z, Xu C, Sun Z, Li J, Liu J, Tian Y, Li H (2021) A Nanometer-Sized Graphite/Boron-Doped Diamond Electrochemical Sensor for Sensitive Detection of Acetaminophen. ACS Omega 6(9):6326–6334. https://doi.org/10.1021/acsomega.0c06141
Gowthaman NS, Lim HN, Shankar S (2020) Electrochemical Scaffold Based on Silver Phosphate Nanoparticles for the Quantification of Acetaminophen in Body Fluids and Pharmaceutical Formulations. ACS Appl Nano Mater 3(2):1213–1222. https://doi.org/10.1021/acsanm.9b01959
Nooshabadi MS, Maleh HK, Javazmi FT (2019) Fabrication of an electroanalytical sensor for determination of deoxyepinephrine in the presence of uric acid using CuFe2O4 nanoparticle/ionic liquid amplified sensor. J Electrochem Soc 166(6):H218–H223. https://doi.org/10.1149/2.1261906jes
Wang X, Yang N, Wan Q, Wang X (2007) Catalytic capability of poly(malachite green) films based electrochemical sensor for oxidation of dopamine. Sens. Actuators B Chem 128(1):83–90. S0925400507003772. https://doi.org/10.1016/j.snb.2007.05.036
Funding
The financial support from the Royal Society and UK aid (ROYAL SOCIETY–FCDO Grant AQ150029-ACBI program) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kemmegne-Mbouguen, J.C., Tchoumi, F.P. Synthesis of nanozeolites type A and X from quartz-rich Cameroonian kaolin: application to the modification of carbon paste electrode for acetaminophen and epinine electrochemical sensing. J Solid State Electrochem 27, 939–953 (2023). https://doi.org/10.1007/s10008-022-05355-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05355-z