Abstract
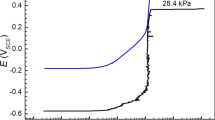
The pitting and crevice corrosion behaviors of 2205 stainless steel were studied in the concentrated seawater at 72 °C, i.e., the simulated low-temperature multi-effect distillation environments, during 11,550 h of immersion by using electrochemical measurement techniques and surface analysis methods. The corrosion evolution successively shows passive, transient, and active corrosion stages. In comparison with the non-creviced specimens, the creviced specimens show insignificant difference in the corrosion potential, but the much lower polarization resistance values in the whole immersion process and shorter induction time for the transition of passive to active state. Crevice corrosion is easier to occur than pitting corrosion in hot concentrated seawater. The corrosion depth is deeper for the crevice than the pits, but is still very shallow. The pitting and crevice corrosion changes from charge transfer control to mixed control of charge transfer and diffusion process in the long-term immersion conditions. The diffusion process dominates the crevice corrosion since about 10,200 h. The ferrite phase dissolves preferentially in the crevice.












Similar content being viewed by others
References
Khawaji AD, Kutubkhanah IK, Wie J-M (2008) Advances in seawater desalination technologies. Desalination 221(1-3):47–69
Elimelech M, Phillip WA (2011) The future of seawater desalination: energy, technology, and the environment. Science 333(6043):712–717
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452(7185):301–310
Matsuura T (2001) Progress in membrane science and technology for seawater desalination—a review. Desalination 134(1-3):47–54
Schiermeier Q (2008) Water: purification with a pinch of salt. Nature 452(7185):260–261
Lee KP, Arnot TC, Mattia D (2011) A review of reverse osmosis membrane materials for desalination—development to date and future potential. J Membr Sci 370(1-2):1–22
Hassan AM, Malik AU (1989) Corrosion resistant materials for seawater RO plants. Desalination 74:157–170
Barchiche C, Deslouis C, Festy D, Gil O, Refait P, Touzain S, Tribollet B (2003) Characterization of calcareous deposits in artificial seawater by impedance techniques: 3—deposit of CaCO3 in the presence of Mg (II). Electrochim Acta 48(12):1645–1654
Laycock N, Stewart J, Newman R (1997) The initiation of crevice corrosion in stainless steels. Corros Sci 39(10-11):1791–1809
Felloni L, Fratesi R, Ruggeri O, Sambogna G (1985) Pitting and crevice corrosion potentials of solar panel stainless steels in seawater and 0.6 M NaCl. Corrosion 41(3):169–177
Hultquist G, Leygraf C (1980) Surface composition of a type 316 stainless steel related to initiation of crevice corrosion. Corrosion 36(3):126–129
Bottoli F, Jellesen MS, Christiansen TL, Winther G, Somers MAJ (2018) High temperature solution-nitriding and low-temperature nitriding of AISI 316: effect on pitting potential and crevice corrosion performance. Appl Surf Sci 431:24–31
Wilde BE, Williams E (1971) The relevance of accelerated electrochemical pitting tests to the long-term pitting and crevice corrosion behavior of stainless steels in marine environments. J Electrochem Soc 118(7):1057–1062
Frankenthal R, Pickering H (1972) On the mechanism of localized corrosion of iron and stainless steel II. Morphological studies. J Electrochem Soc 119(10):1304–1310
Ijsseling F (1980) Electrochemical methods in crevice corrosion testing: report prepared for the European federation of corrosion working party ‘Physico-chemical testing methods of corrosion: Fundamentals and applications’. Br Corros J 15(2):51–69
Kruger J, Rhyne K (1982) Current understanding of pitting and crevice corrosion and its application to test methods for determining the corrosion susceptibility of nuclear waste metallic containers. Nucl Chem Waste Manag 3(4):205–227
Oldfield JW (1987) Test techniques for pitting and crevice corrosion resistance of stainless steels and nickel-base alloys in chloride-containing environments. Int Mater Rev 32(1):153–172
McCafferty E (1990) A competitive adsorption model for the inhibition of crevice corrosion and pitting. Chest 137(12):3731–3737
Morales J, Esparza P, Salvarezza R, Gonzalez S (1992) The pitting and crevice corrosion of 304 stainless steel in phosphate-borate buffer containing sodium chloride. Corros Sci 33(10):1645–1651
Pardo A, Otero E, Merino MC, López MD, Utrilla MV, Moreno F (2000) Influence of pH and chloride concentration on the pitting and crevice corrosion behavior of high-alloy stainless steels. Corrosion 56(4):411–418
Li MC, Zeng CL, Luo SZ, Shen JN, Lin HC, Cao CN (2003) Electrochemical corrosion characteristics of type 316 stainless steel in simulated anode environment for PEMFC. Electrochim Acta 48(12):1735–1741
Li MC, Royer M, Stien D, Lecante A, Roos C (2008) Inhibitive effect of sodium eperuate on zinc corrosion in alkaline solutions. Corros Sci 50(7):1975–1981
Oldfield J, Sutton W (1978) Crevice corrosion of stainless steels: II. Experimental studies. Br Corros J 13(3):104–111
Xin SS, Li MC (2014) Electrochemical corrosion characteristics of type 316L stainless steel in hot concentrated seawater. Corros Sci 81:96–101
Serdar M, Žulj LV, Bjegović D (2013) Long-term corrosion behaviour of stainless reinforcing steel in mortar exposed to chloride environment. Corros Sci 69:149–157
Dawson J, Ferreira M (1986) Crevice corrosion on 316 stainless steel in 3% sodium chloride solution. Corros Sci 26(12):1027–1040
Zeng H, Yang Y, Xu R, Xin S, Li M (2019) Pitting corrosion resistance of sensitized type 2205 duplex stainless steel in hot concentrated seawater. J Solid State Electrochem 23(10):2793–2801
ASTM (2015) G78-15 Standard guide for crevice corrosion testing of iron-base and nickel-base stainless alloys in seawater and other chloride-containing aqueous environments. West Conshohocken, PA
Park J, Macdonald D (1983) Impedance studies of the growth of porous magnetite films on carbon steel in high temperature aqueous systems. Corros Sci 23(4):295–315
Budhiraja P, Fares AA (2008) Studies of scale formation and optimization of antiscalant dosing in multi-effect thermal desalination units. Desalination 220(1-3):313–325
Macdonald DD (2011) The history of the Point Defect Model for the passive state: a brief review of film growth aspects. Electrochim Acta 56(4):1761–1772
Abreu C, Cristóbal M, Losada R, Nóvoa X, Pena G, Pérez M (2006) Long-term behaviour of AISI 304L passive layer in chloride containing medium. Electrochim Acta 51(8-9):1881–1890
Tsai W-T, Chen J-R (2007) Galvanic corrosion between the constituent phases in duplex stainless steel. Corros Sci 49(9):3659–3668
Yang Y, Zeng H, Xin S, Hou X, Li M (2020) Electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater under vacuum conditions. Corros Sci 165:108383
Zeng H, Yang Y, Zeng M, Li M (2021) Effect of dissolved oxygen on electrochemical corrosion behavior of 2205 duplex stainless steel in hot concentrated seawater. J Mater Sci Technol 66:177–185
Urquidi-Macdonald M, Real S, Macdonald DD (1990) Applications of Kramers—Kronig transforms in the analysis of electrochemical impedance data—III. Stability and linearity. Electrochim Acta 35(10):1559–1566
Bäck G, Singh PM (2004) Susceptibility of stainless steel alloys to crevice corrosion in ClO2 bleach plants. Corros Sci 46(9):2159–2182
Wu X, Liu Y, Sun Y, Dai N, Li J, Jiang Y (2021) A discussion on evaluation criteria for crevice corrosion of various stainless steels. J Mater Sci Technol 64:29–37
Orlikowski J, Jazdzewska A, Mazur R, Darowicki K (2017) Determination of pitting corrosion stage of stainless steel by galvanodynamic impedance spectroscopy. Electrochim Acta 253:403–412
Ma G, Wu G, Shi W, Xiang S, Chen Q, Mao X (2020) Effect of cold rolling on the corrosion behavior of Ta-4W alloy in sulphuric acid. Corros Sci 176:108924
Ramanathan R, Voorhees PW (2019) Morphological stability of steady-state passive oxide films. Electrochim Acta 303:299–315
Macdonald DD, Lei X (2016) Theoretical interpretation of anion size effects in passivity breakdown. J Electrochem Soc 163(13):C738–C744
Macdonald DD (1992) The point defect model for the passive state. J Electrochem Soc 139(12):3434–3449
Oldfield J, Sutton W (1978) Crevice corrosion of stainless steels: I. A mathematical model. Br Corros J 13(1):13–22
Heppner K, Evitts R, Postlethwaite J (2004) Effect of the crevice gap on the initiation of crevice corrosion in passive metals. Corrosion 60(8):718–728
Ning F, Tan J, Wu X (2020) Effects of 405 stainless steel on crevice corrosion behavior of Alloy 690 in high-temperature pure water. J Mater Sci Technol 47:76–87
Yang YZ, Jiang YM, Li J (2013) In situ investigation of crevice corrosion on UNS S32101 duplex stainless steel in sodium chloride solution. Corros Sci 76:163–169
Shojaei E, Mirjalili M, Moayed MH (2019) The influence of the crevice induced IR drop on polarization measurement of localized corrosion behavior of 316 L stainless steel. Corros Sci 156:96–105
Zadorozne NS, Giordano C, Rodríguez MA, Carranza RM, Rebak RB (2012) Crevice corrosion kinetics of nickel alloys bearing chromium and molybdenum. Electrochim Acta 76:94–101
Cheng X, Wang Y, Li X, Dong C (2018) Interaction between austein-ferrite phases on passive performance of 2205 duplex stainless steel. J Mater Sci Technol 34(11):2140–2148
Lo IH, Fu Y, Lin C-J, Tsai W-T (2006) Effect of electrolyte composition on the active-to-passive transition behavior of 2205 duplex stainless steel in H2SO4/HCl solutions. Corros Sci 48(3):696–708
Femenia M, Pan J, Leygraf C (2002) In situ local dissolution of duplex stainless steels in 1M H2SO4 + 1M NaCl by Electrochemical Scanning Tunneling Microscopy. J Electrochem Soc 149(6):B187–B197
Laitinen A, Hänninen H (1996) Chloride-induced stress corrosion cracking of powder metallurgy duplex stainless steels. Corrosion 52(4):295–306
Lindgren M, Huttunen-Saarivirta E, Peltola H, Romu J, Sarikka T, Hänninen H, Pohjanne P (2018) Crevice corrosion of stainless steels 904 L, 2205, and 2507 in high-temperature sulfuric acid solution containing chlorides: Influence of metal cations. Corrosion 74(2):225–240
Oldfield J, Sutton W (1980) New technique for predicting the performance of stainless steels in seawater and other chloride-containing environments. Br Corros J 15(1):31–34
Symniotis E (1990) Galvanic effects on the active dissolution of duplex stainless steels. Corrosion 46(1):2–12
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. U1960103 and 51571139).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, H., Yang, Y., Liu, L. et al. Pitting and crevice corrosion evolution characteristics of 2205 duplex stainless steel in hot concentrated seawater. J Solid State Electrochem 25, 1555–1565 (2021). https://doi.org/10.1007/s10008-021-04935-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-04935-9