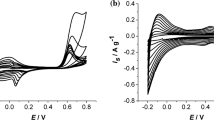
Abstract
The methods of silicotungstic acid (SiW) immobilization on conducting substrates were studied. For SiW immobilization by codeposition, poly-o-phenylenediamine (PPD) redox polymer was used. The most effective codeposition of SiW and PPD was demonstrated on a graphene oxide (GO) film. Meanwhile, GO is reduced to form RGO-PPD-SiW electroactive composite. The structure of the novel material was evidenced by cyclic voltammetry and IR and Raman spectra. PPD-SiW and RGO-PPD-SiW composites were studied by impedance spectroscopy, where an equivalent circuit was proposed. Film resistance Rf was shown to decrease in the series of PPD → RGO-PPD → RGO-PPD-SiW. Further, RGO-PPD-SiW has better transfer properties (bulk film diffusion rate). This enabled suggesting that the composite has better electrocatalytic properties than PPD and RGO-PPD as was evidenced for example of [Fe(CN)6]4−/3− redox transfer on the electrode coated with the novel material.













Similar content being viewed by others
Notes
Avtocom, Moscow, http://www.membrans.ru/
Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Russia
Produced by Electrochemical Instruments (Chernogolovka, Moscow Region). http://potentiostat.ru
References
Peresypkina EV, Virovets AV, Adonin SA, Abramov PA, Rogachev AV, Sinkevich PL, Korenev VS, Sokolov MN (2014) Crystal structure of two salts derived from paratungstate in the [H2W12O42]10− anion. J Struct Chem 55:295–298
Prudent R, Moucadel V, Laudet B, Barette C, Lafanechère L, Hasenknopf B, Li J, Bareyt S, Lacôte E, Thorimbert S, Malacria M, Gouzerh P, Cochet C (2008) Identification of polyoxometalates as nanomolar noncompetitive inhibitors of protein kinase. CK2 Chem Biol 15(7):83–692
Rhule JT, Hill CL, Judd DA (1998) Polyoxometalates in medicine. Chem Rev 98:327–357
Li T, Zhang Z, Li W, Liu C, Zhou H, An L (2016) Electrospinning preparation, characterization, and enhanced photocatalytic activity of an silicotungstic acid (H4SiW12O40)/poly(vinyl alcohol)/poly(methyl methacrylate) composite nanofiber membrane. J Appl Polym Sci 133:43193
Herrmann S, Ritchie C, Streb C (2015) Polyoxometalate – conductive polymer composites for energy conversion, energy storage and nanostructured sensors. Dalton Trans 44:7092–7104
Tamirat AG, Guan X, Liu J, Luo J, Xia Y (2020) Redox mediators as charge agents for changing electrochemical reactions. Chem Soc Rev 49:7454
Sadakane M, Steckhan E (1998) Electrochemical properties of polyoxometalates as electrocatalysts. Chem Rev 98(1):219–237
Guo D, Zhou C, Tan L, Ma H, He R, Pang H, Wang X (2020) Electrochemical ascorbic acid sensor of composite film based on Keggin-type Vanadium-substituted polyoxometalates decorated with graphene and Ru (bpy)32+. Colloids Surf A 592:124550
Liu L, Wang B, Du Y, Borgna A (2015) Supported H4SiW12O40/Al2O3 solid acid catalysts for dehydration of glycerol to acrolein: avolution of catalyst structure and performance with calcination temperature. Appl Catal A: Gen 489:32–41
Pisarevskaya EY, Efimov ON (2019) Graphene oxide as a basis for molecular design. Prot Met Phys Chem Surf 55:468–472
Wang B, Dong S (1996) Electrochemical study of isopoly- and heteropoly-oxometalates film modified microelectrodes —vi preparation and redox properties of 12-molybdophosphoric acid and 12-molybdosilicic acid modified carbon fiber microelectrodes. Electrochim Acta 41:895–902
Pisarevskaya EY, Makarychev YB, Dremova NN, Girina GP, Efimov ON (2018) A study of a novel nanocomposite material based on reduced graphene oxide and poly(o-phenylenediamine). Prot Met Phys Chem Surf 54:393–401
Pisarevskaya EY, Rychagov AY, Gorbunov AM, Averin AA, Makarychev YB, Efimov ON (2018) Synthesis of nanostructured conducting composite films based on reduced graphene oxide and o-phenylenediamine. Synth Met 243:1–7
Pisarevskaya EY, Kolesnichenko II, Averin AA, Gorbunov AM, Efimov ON (2020) A novel multifunctional composite based on reduced graphene oxide, poly-o-phenylenediamine and silicotungstic acid. Synth Met 270:116596
Aparicio-Anglès X, Clotet A, Bo C, Poblet JM (2011) Towards the computational modelling of polyoxoanions on metal surfaces: IR spectrum characterisation of [SiW12O40]4− on Ag(111). Phys Chem Chem Phys 13(33):15143–15147
Gurunathan S, Han JW, Kim E, Park JH, Kim J (2015) Reduction of graphene oxide by resveratrol: a novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int J Nanomed 10:2951–2969
Pisarevskaya EYu, Averin AA, Makarychev YB, Efimov ON (2019) Modifying graphene oxide and poly-o-phenylene diamine-graphene oxide composite with palladium particles. 257:116153
Ucar A, Findik M, Gubbuk IH, Kocak N, Bingol H (2017) Catalytic degradation of organic dye using reduced graphene oxide–polyoxometalate nanocomposite. Mater Chem Phys 196:21–28
Bilal S, Shah A-u-HA, Holze R (2011) Spectroelectrochemistry of poly(o-phenylenediamine): polyaniline-like segments in the polymer structure. Electrochim Acta 56(9):3353–3358
Wu L-L, Luo J, Lin Z-H (1996) Spectroelectrochemical studies of poly-o-phenylenediamine part 1 In situ resonance Raman spectroscopy. J Electroanal Chem 417(1-2):53–58
Martinusz K, Láng G, Inzelt G (1997) Impedance analysis of poly(o-phenylenediamine) electrodes. J Electroanal Chem 433(1-2):1–8
Láng GG, Ujvári M, Inzelt G (2004) Analysis of the impedance spectra of Pt|poly(o-phenylenediamine) electrodes - hydrogen adsorption and the brush model of the polymer films. J Electroanal Chem 572(2):283–297
Orasem ME, Tribollet B (2008) Electrochemical impedance spectroscopy. Wiley, The Electrochemical Society series ISBN 978-0-470-04140-6
Levi MD, Pisarevskaya EY (1992) Cyclic and steady-state voltammetric studies of the mechanism and kinetics of some solute inorganic redox-species reactions at a gold electrode covered with poly-o-phenylenediamine. Electrochim Acta 37:635–641
Acknowledgments
The work was performed within the State Program of IPCE RAS and IPCP RAS (no. of state registration are АААА-А19-119041890032-6 and АААА-А19-119071190044-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 596 kb)
Rights and permissions
About this article
Cite this article
Pisarevskaya, E.Y., Klyuev, A.L., Averin, A.A. et al. One-pot electrosynthesis and physicochemical properties of multifunctional material based on graphene oxide, poly-o-phenylenediamine, and silicotungstic acid. J Solid State Electrochem 25, 859–868 (2021). https://doi.org/10.1007/s10008-020-04859-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04859-w