Abstract
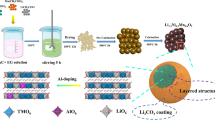
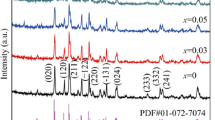
The poor rate performance and low discharge capacity of LiCoO2 limit its applications. Therefore, in this work, we synthesized Li1-xMxCoO2 (M = Na, Zr, Nb) via solid-phase synthesis to improve its properties. X-ray diffraction (XRD) results suggest that the doping elements were successfully doped into LiCoO2. The electrochemical properties showed that the samples doped with the high-valence elements Zr and Nb had a higher capacity, better cycle stability, and better rate performance than those doped with the low-valence element Na. In particular, the capacity retention of LiCoO2, Li0.97Na0.03CoO2, Li0.99Zr0.01CoO2, and Li0.99Nb0.01CoO2 was 68, 42, 85, and 87%, respectively, after 80 cycles at a rate of 10 C at 55 °C. However, doping of Zr and Nb into the Li+ site of LiCoO2 will reduce the content of Li+. And, less Li+ extracted in the cathode material resulting in low discharge capacity under low current density. The larger radius of Na+ is incorporated into the Li slab and enlarged the interlayer spacing of the (003) plane. The larger (003) interplanar spacing can significantly facilitate the lithium diffusion and is also favorable to the rate capability. The differential scanning calorimetry (DSC) and thermogravimetric (TG) analysis results demonstrated that the Zr-doped and Nb-doped LiCoO2 had a higher thermal stability in the charged state than the Na-doped LiCoO2. Additionally, the resistances of the Zr-doped and Nb-doped electrodes were much lower than that of the undoped electrode. Our research results indicate that doping with high-valence elements is a very effective strategy for optimizing the electrochemical performance of LiCoO2 and that this method can also be extended to other cathode materials.

ᅟ









Similar content being viewed by others
References
Wang Z, Wu C, Liu L, Wu F, Chen L, Huang X (2002) Electrochemical evaluation and structural characterization of commercial LiCoO2 surfaces modified with MgO for lithium-ion batteries. J Electrochem Soc 149(4):A466–A471
Isobe M, Ueda Y (2004) Synthesis, structure and physical properties of spinel solid solutions Mg2TiO4—MgTi2O4. Cheminform 383(2):85–88
Wang YH, Chen L, Wang YG, Xia YY (2015) Cycling stability of spinel LiMn2O4 with different particle sizes in aqueous electrolyte. Electrochim Acta 173(10):178–183
Ji Y, Zhang P, Lin M, Zhao W, Zhang Z, Zhao Y, Yang Y (2017) Toward a stable electrochemical interphase with enhanced safety on high-voltage LiCoO2 cathode: a case of phosphazene additives. J Power Sources 359:391–399
Rangaswamy P, Suresh GS, Kittappa MM (2016) A new tavorite LiTiPO4F electrode material for aqueous rechargeable lithium ion battery. J Solid State Electrochem 20(10):2619–2631
Chen M, Zhao E, Chen D, Wu M, Han S, Huang Q, Yang L, Xiao X, Hu Z (2017) Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg Chem 56(14):8355–8362
Chen M, Zhao E, Yan Q, Hu Z, Xiao X, Chen D (2016) The effect of crystal face of Fe2O3 on the electrochemical performance for lithium-ion batteries. Sci Rep 6(1):29381
Zhao E, Chen M, Hu Z, Xiao X, Chen D (2016) Layered/layered homostyructure ion conductor coating strategy for high performance lithium ion batteries. Electrochim Acta 208:64–70
Shen B, Zuo P, Fan P, Yang J, Yin G, Ma Y, Cheng X, Du C, Gao Y (2016) Improved electrochemical performance of NaAlO2-coated LiCoO2 for lithium-ion batteries. J Solid State Electrochem 21(5):1195–1201
Swiderska-Mocek A, Lewandowski A (2017) Kinetics of Li-ion transfer reaction at LiMn2O4 , LiCoO2 , and LiFePO4 cathodes. J Solid State Electrochem 21(5):1365–1372
Rao MM, Liebenow C, Jayalakshmi M, Wulff H, Guth U, Scholz F (2001) High-temperature combustion synthesis and electrochemical characterization of LiNiO2, LiCoO2 and LiMn2O4 for lithium-ion secondary batteries. J Solid State Electrochem 5(5):348–354
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414(6861):359–367
Ohzuku T, Ueda A (1994) Solid-state redox reactions of LiCoO2 (R[bar 3]m) for 4 volt secondary lithium cells. J Electrochem Soc 141(11):2972–2977
Whittingham MS (2004) Lithium batteries and cathode materials. Cheminform 35(50):4271–4301
Zuo D (2017) Comparative study of the electrochemical behaviors for LiCoO2 electrode coated with two different Al2O3 coating layer. Int J Electrochem Sci 12(6):5044–5057
Sheng S, Chen G, Hu B, Yang R, Xu Y (2017) Al2O3-surface modification of LiCoO2 cathode with improved cyclic performance. J Electroanal Chem 797:59–67
Liang DD, Xiang HF, Liang X, Cheng S, Chen CH (2017) Spinel MgAl2O4 modification on LiCoO2 cathode materials with the combined advantages of MgO and Al2O3 modifications for high-voltage lithium-ion batteries. RSC Adv 7(12):6809–6817
Taguchi N, Sakaebe H, Akita T, Tatsumi K, Ogumi Z (2014) Characterization of surface of LiCoO2 modified by Zr oxides using analytical transmission electron microscopy. J Electrochem Soc 161(10):1521–1526
Hwang BJ, Chen CY, Cheng MY, Santhanam R, Ragavendran K (2010) Mechanism study of enhanced electrochemical performance of ZrO2-coated LiCoO2 in high voltage region. J Power Sources 195(13):4255–4265
Taguchi N, Akita T, Tatsumi K, Sakaebe H (2016) Characterization of MgO-coated-LiCoO2 particles by analytical transmission electron microscopy. J Power Sources 328:161–166
Tukamoto H, West AR (1997) Electronic conductivity of LiCoO2 and its enhancement by magnesium doping. Cheminform 28(52):3164–3168
Shim JH, Lee S, Park SS (2014) Effects of MgO coating on the structural and electrochemical characteristics of LiCoO2 as cathode materials for lithium ion battery. Chem Mater 26(8):2537–2543
Zhang J, Gao R, Sun L, Zhang H, Hu Z, Liu X (2016) Unraveling the multiple effects of Li2ZrO3 coating on the structural and electrochemical performances of LiCoO2 as high-voltage cathode materials. Electrochim Acta 209:102–110
Stoyanova R, Zhecheva E, Zarkova L (1994) Effect of Mn-substitution for Co on the crystal structure and acid delithiation of LiMnyCo1− yO2 solid solutions. Solid State Ionics 73(3–4):233–240
Buyukburc A, Aydinol MK (2014) Effect of Cr and Mo doping on the electrochemical properties of freeze-dried LiCoO2. Int J Mater Res 105(10):983–991
Sathiyamoorthi R, Chandrasekaran R, Gopalan A, Vasudevan T (2008) Synthesis and electrochemical performance of high voltage cycling LiCo0.8M0.2O2 (M = Mg, Ca, Ba) as cathode material. Mater Res Bull 43(6):1401–1411
Xie H, Du K, Hu G, Peng Z, Cao Y (2016) The role of sodium in LiNi0.8Co0.15Al0.05O2 cathode material and its electrochemical behaviors. J Phys Chem C 120(6):3235–3241
Kaneda H (2017) Improving the cycling performance and thermal stability of LiNi0.6Co0.2Mn0.2O2 cathode materials by Nb-doping and surface modification. Int J Electrochem Sci 12(6):4640–4653
Gummow RJ, Thackeray MM, David W, Hull S (1992) Structure and electrochemistry of lithium cobalt oxide synthesised at 400°C. Mater Res Bull 27(3):327–337
Zhao E, Chen M, Chen D, Xiao X, Hu Z (2015) A versatile coating strategy to highly improve the electrochemical properties of layered oxide LiMO2 (M = Ni0.5Mn0.5 and Ni1/3Mn1/3Co1/3). Acs Appl Mater Inter 7(49):27096–27105
Wang QS, Sun JH, Chen CH, Zhou XM (2008) Thermal properties and kinetics study of charged LiCoO2 by TG and C80 methods. J Therm Anal Calorim 92(2):563–566
Wang Q, Sun J, Chen D, Chen C (2009) Comparison of the thermal decomposition kinetics for charged LiMn2O4 by TG and C80 methods. J Alloy Compd 468(1–2):477–481
Wang Q, Sun J, Yao X, Chen C (2005) Thermal stability of LiPF6/EC + DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim Acta 437(1–2):12–16
Sun YK, Cho SW, Myung ST, Amine K, Prakash J (2007) Effect of AlF3 coating amount on high voltage cycling performance of LiCoO2. Electrochim Acta 53(2):1013–1019
Wang Z, Wang Z, Guo H, Peng W, Li X, Yan G, Wang J (2015) Mg doping and zirconium oxyfluoride coating co-modification to enhance the high-voltage performance of LiCoO2 for lithium ion battery. J Alloy Compd 621:212–219
Martha SK, Nanda J, Veith GM, Dudney NJ (2012) Electrochemical and rate performance study of high-voltage lithium-rich composition: Li1.2Mn0.525Ni0.175Co0.1O2. J Power Sources 199(1):220–226
Zhao E, Liu X, Zhao H, Xiao X, Hu Z (2015) Ion conducting Li2SiO3-coated lithium-rich layered oxide exhibiting high rate capability and low polarization. Chem Commun 51(44):9093–9096
Acknowledgments
This work was supported by the Beijing Nova Program (Z141103001814065), the State Key Project of Fundamental Research (2014CB931900 and 2012CB932504), the Youth Innovation Promotion Association CAS (2016152), the National Natural Science Foundation of China (U1507106 and U1507114), and the Natural Science Foundation of Qinghai Province (2015-ZJ-935Q).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Highlights
1. We synthesized Li1-xMxCoO2 (M = Na, Zr, Nb) by solid-phase synthesis successfully.
2. All samples have a well layered structure.
3. Large radius ions can broaden the interlayer spacing of Li layer.
4. Li1-xMxCoO2 (M = Zr, Nb) have high thermal stability at the charged state.
5. Doping Nb5+ and Zr4+ into Li+ sites of LiCoO2 demonstrate better rate performance.
Rights and permissions
About this article
Cite this article
Wu, K., Li, Q., Chen, M. et al. Improving the cycling performance, rate capacity, and thermal stability of LiCoO2 by doping high-valence ions into the Li+ site. J Solid State Electrochem 22, 3725–3734 (2018). https://doi.org/10.1007/s10008-018-4046-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4046-4