Abstract
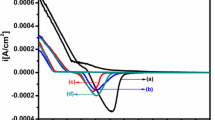
Gd3+ (gadolinium)-doped ZnSe thin films (1 to 5 mol%) are grown onto indium-doped tin oxide (ITO) glass substrate by single-step electrochemical deposition process. X-ray diffraction analysis confirms the formation of hexagonal wurtzite structure with preferred growth orientation along (101) plane. A new antistructural modeling for describing active surface centers for ZnSe:Gd system is discussed for the first time. The new antistructural modeling shows that the dissolution of Gd cations increases the concentration of surface active centers \( {\mathrm{Gd}}_{\mathrm{Zn}}^{\bullet } \) and \( {\mathrm{V}}_{\mathrm{Zn}}^{\prime \prime } \), which are located in the cationic sublattice. The surface morphology of thin films investigated using scanning electron microscopy reveals some agglomeration of grains with significant changes in particle size with varying Gd3+ concentrations. UV-vis and photoluminescence studies indicate a blue shift due to the incorporation of Gd3+ into ZnSe host lattice. Electrochemical impedance spectroscopy and photoelectrochemical measurements reveal that the 3 mol% Gd3+-doped ZnSe thin film possesses low charge transfer resistance (25.42 Ω) and faster migration of photoinduced electrons, resulting in high conductivity. Therefore, the optimum doping concentration, 3 mol% Gd3+-doped ZnSe, offers a positive synergistic effect for photoelectrochemical devices.










Similar content being viewed by others
References
Xie R, Li Y, Zhang X, Liu H (2015) Low-cost environmentally friendly synthesis, structural and spectroscopic properties of Fe:ZnSe colloidal nanocrystals. J Alloys Compd 621:396–403. https://doi.org/10.1016/j.jallcom.2014.09.217
Yadav K, Jaggi N (2015) Effect of Ag doping on structural and optical properties of ZnSe nanophosphors. Mater Sci Semicond Process 30:376–380. https://doi.org/10.1016/j.mssp.2014.09.044
Prabukanthan P, Harichandran G (2014) Electrochemical deposition of n-type ZnSe thin film buffer layer for solar cells. J Electrochem Soc 14:D736–D741. https://doi.org/10.1149/2.0261414jes
Poornaprakash B, Chalapathi U, Reddeppa M, Park SH (2016) Effect of Gd doping on the structural, luminescence and magnetic properties of ZnS nanoparticles synthesized by the hydrothermal method. Superlattice Microst 97:104–109. https://doi.org/10.1016/j.spmi.2016.06.013
Prabukanthan P, Rajesh Kumar T, Harichandran G (2017) Influence of various complexing agents on structural, morphological, optical and electrical properties of electrochemically deposited ZnSe thin films. J Mater Sci Mater Electron 28(19):14728–14737. https://doi.org/10.1007/s10854-017-7341-4
Boulon G (2012) Fifty years of advances in solid-state laser materials. Opt Mater 34(3):499–512. https://doi.org/10.1016/j.optmat.2011.04.018
Rajesh Kumar T, Prabukanthan P, Harichandran G, Theerthagiri J, Chandrasekaran S, Madhavan J (2017) Optical, magnetic, and photoelectrochemical properties of electrochemically deposited Eu3+-doped ZnSe thin films. Ionics 23(9):2497–2507. https://doi.org/10.1007/s11581-017-2090-1
Colibaba GV, Goncearenco EP, Nedeoglo DD, Nedeoglo ND (2015) Infrared photoluminescence of ZnSe:Gd crystals. J Lumin 158:451–455. https://doi.org/10.1016/j.jlumin.2014.10.048
Nasieka IU, Boyko M, Strelchuk V, Kovalenko N, Gerasimenko A, Starzhinskiy N, Zhukov A, Zenya I, Sofronov D (2014) Optical characterization of Er-doped ZnSe for scintillation applications. Opt Mater 38:272–277. https://doi.org/10.1016/j.optmat.2014.10.052
Jonker BT, Peterson LD, Krebs JJ (1993) Growth and characterization of a new diluted magnetic semiconductor, Zn1-x Eu xSe. J Appl Phys 73(10):5742–5744. https://doi.org/10.1063/1.353610
Sadowski J, Dynowska E, Szamota-Sadowska K, Przedpelski W, Sitarek P, Swiarek K (1996) Properties of MBE grown CdYbTe and ZnYbTe on GaAs (100) substrates. J Cryst Growth 159(1-4):1075–1079. https://doi.org/10.1016/0022-0248(95)00841-1
Lohar GM, Jadhav ST, Takale MV, Patil RA, Mab YR, Rath MC, Fulari VJ (2015) Photoelectrochemical cell studies of Fe2+ doped ZnSe nanorods using the potentiostatic mode of electrodeposition. J Colloid Interface Sci 458:136–146. https://doi.org/10.1016/j.jcis.2015.07.046
Moses Ezhil Raj A, Mary Delphine S, Sanjeeviraja C, Jayachandran M (2010) Growth of ZnSe thin layers on different substrates and their structural consequences with bath temperature. Physica B 405(10):2485–2491. https://doi.org/10.1016/j.physb.2010.03.019
Metina H, Durmus S, Erat S, Ari M (2011) Characterization of chemically deposited ZnSe/SnO2/glass films: influence of annealing in Ar atmosphere on physical properties. Appl Surf Sci 257(15):6474–6480. https://doi.org/10.1016/j.apsusc.2011.02.047
Wei A, Zhao X, Liu J, Zhao Y (2013) Investigation on the structure and optical properties of chemically deposited ZnSe nanocrystalline thin films. Physica B 410:120–125. https://doi.org/10.1016/j.physb.2012.10.031
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32(5):751–767. https://doi.org/10.1107/S0567739476001551
Tatarchuk TR, Bououdina M, Paliychuk ND (2017) Structural characterization and antistructure modeling of cobalt-substituted zinc ferrites. J Alloys Compd 694:777–791. https://doi.org/10.1016/j.jallcom.2016.10.067
Tatarchuk T, Bououdina M, Macyk W, Shyichuk O, Paliychuk N, Yaremiy I, Al-Najar B, Pacia M (2017) Structural, optical, and magnetic properties of Zn-doped CoFe2O4 nanoparticles. Nanoscale Res Lett 12(1):141–152. https://doi.org/10.1186/s11671-017-1899-x
Kurta SA, Mykytyn IM, Tatarchuk TR (2014) Structure and the catalysis mechanism of oxidative chlorination in nanostructural layers of a surface of alumina. Nanoscale Res Lett 9(1):357–365. https://doi.org/10.1186/1556-276X-9-357
Kebalo GI, Lisnyak SS, Tatarchuk TR (2004) Mechanism of reactions in hematite-lithium carbonate system. Ukrainskij Khimicheskij Zhurnal 70:94–97
Horichok V, Ya H, Prokopiv VV, Pylyponiuk MA (2016) Semiempirical energies of vacancy formation in semiconductors. Ukr J Phys 61(11):992–1007. https://doi.org/10.15407/ujpe61.11.0992
Marfaing Y (1996) Fundamental studies on compensation mechanisms in II–VI compounds. J Cryst Growth 161(1-4):205–213. https://doi.org/10.1016/0022-0248(95)00641-9
Colibaba G, Caraman M, Evtodiev I, Evtodiev S, Goncearenco E, Nedeoglo D, Nedeoglo N (2014) Influence of annealing medium on photoluminescence and optical properties of ZnSe:Cr crystals. J Lumin 145:237–243. https://doi.org/10.1016/j.jlumin.2013.07.018
Ji Y, Cao J, Jiang L, Zhang Y, Yi Z (2014) g-C3N4/BiVO4 composites with enhanced and stable visible light photocatalytic activity. J Alloys Compd 590:9–14. https://doi.org/10.1016/j.jallcom.2013.12.050
Natarajan C, Sharon M, Clment CL, Spallart MN (1994) Electrodeposition of zinc selenide. Thin Solid Films 237(1-2):118–123. https://doi.org/10.1016/0040-6090(94)90247-X
Lohar GM, Shinde SK, Rath MC, Fulari VJ (2014) Structural, optical, photoluminescence, electrochemical and photoelectrochemical properties of Fe doped ZnSe hexagonal nanorods. Mater Sci Semicond Process 26:548–554. https://doi.org/10.1016/j.mssp.2014.05.047
Prabukanthan P, Rajesh Kumar T, Harichandran G (2015) Effect of Sm3+ on the structural, optical, magnetic and electrical properties of electrochemical deposition of ZnSe thin films. Mater Res Express 2(9):096102. https://doi.org/10.1088/2053-1591/2/9/096102
Ali Z, Cao C, Khan WS, Butt FK, Hussain S, Mahmood T, Nabi G, Usman Z (2012) Simultaneous growth of ZnSe cactus-like structures and novel microflowers of selenium. J Alloys Compd 513:620–625. https://doi.org/10.1016/j.jallcom.2011.11.028
Yadav K, Dwivedi Y, Jaggi N (2015) Structural and optical properties of Ni doped ZnSe nanoparticles. J Lumin 158:181–187. https://doi.org/10.1016/j.jlumin.2014.09.025
Wang Y, Liang X, Liu E, Hu X, Fan J (2015) Incorporation of lanthanide (Eu3+) ions in ZnS semiconductor quantum dots with a trapped-dopant model and their photoluminescence spectroscopy study. Nanotechnology 26(37):375601. https://doi.org/10.1088/0957-4484/26/37/375601
Mukherjee P, Sloan RF, Shade CM, Waldeck DH, Petoud S (2013) A postsynthetic modification of II–VI semiconductor nanoparticles to create Tb3+ and Eu3+ luminophores. J Phys Chem C 117(27):14451–14460. https://doi.org/10.1021/jp404947x
Cheng XQ, Ma CY, Yi XY, Yuan F, Xie Y, JM H, BC H, Zhang QY (2016) Structural, morphological, optical and photocatalytic properties of Gd-doped TiO2 films. Thin Solid Films 615:13–18. https://doi.org/10.1016/j.tsf.2016.06.049
Theerthagiri J, Senthil RA, Priya A, Madhavan J, Michael RJV (2014) Photocatalytic and photoelectrochemical studies of visible-light active a-Fe2O3–g-C3N4 nanocomposites. RSC Adv 4(72):38222–38229. https://doi.org/10.1039/C4RA04266B
Acknowledgements
Dr. P. Prabukanthan would like to acknowledge the financial support of Science & Engineering Research Board (SERB)-Empowerment and Equity Opportunities for Excellence in Science [EMEQ] program (F.No.SB/EMEQ-259/2014), Department of Science and Technology (DST), India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajesh Kumar, T., Prabukanthan, P., Harichandran, G. et al. Physicochemical and electrochemical properties of Gd3+-doped ZnSe thin films fabricated by single-step electrochemical deposition process. J Solid State Electrochem 22, 1197–1207 (2018). https://doi.org/10.1007/s10008-017-3865-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3865-z