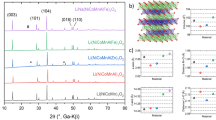
Abstract
The synthesis method of lithiated d-metal oxides using molten formate mixtures as precursors has been developed and the isothermal (800 °C) cross section of pseudo ternary Li–Mn–Co oxide system under ambient oxygen pressure has been investigated by XRD, 7Li NMR, and galvanostatic electrochemical methods. Special attention has been paid to the compositions inside the quadrangle restricted by solid solutions LiCoO2–LiCo0.85Mn0.15O2 with the layered structure of α-NaFeO2 and solid solutions LiMn2O4–LiMnCoO4 with the structure of spinel. It was found that, depending on the composition, three types of equilibrium phases could be formed: spinels Li[Li,Mn,Co]2O4 with a part of Li atoms in octahedral sites, cation-deficit layered compounds Li1 − δ [Co,Mn]O2, and Li2MnO3. Areas of (co)existence of these phases were plotted on the composition plane of the pseudo-ternary Li–Mn–Co system. Electrochemical properties of the compositions inside the quadrangle LiCoO2–LiCo0.85Mn0.15O2–LiMn2O4–LiMnCoO4 are determined by the content and average oxidation number of Mn atoms, which is higher than in the normal spinels Li[Mn,Co]2O4. Thus, the specific capacities of the polyphase compositions are lower in comparison with the binary solid solutions Li[Mn,Co]2O4 or pure LiCoO2.












Similar content being viewed by others
References
Whittingham MS (2004) Chem Rev 104:4271–4301
Tao H, Feng Z, Liu H, Kan X, P. Chen P (2011) Open Mater Sci J 5:204–214
Shukla AK, Prem Kumar T (2008) Curr Sci 94:314–331
Wakihara M, Ikuta H, Uchimoto Y (2002) Ionics 8:329–338
Lee BW (2002) J Power Sources 109:220–226
Suryakala K, Marikkannu KR, Paruthimal Kalaignan G, Vasudevan T (2007) J Solid State Electrochem 11:1671–1677
Makhonina EV, Dubasova VS, Pervov VS, Nikolenko AF, Fialkov AS (2001) Inorg Mater 37:1073–1079
Yanase I, Ohtaki T, Watanabe M (2002) Solid State Ionics 151:189–196
Zhecheva E, Stoyanova R, Gorova M, Lavela P, Tirado JL (2001) Solid State Ionics 140:19–33
Shigemura H, Tabuchi M, Kobayashi H, Sakaebe H, Hirano A, Kageyama H (2002) J Mater Chem 12:1882–1891
Yen-Pei F, Yu-Hsiu S, Lin C-H, Wu S-H (2006) J Mater Sci 41:1157–1164
Franger S, Bach S, Pereira-Ramos JP, Baffier N (2006) J Solid State Electrochem 10:389–396
Yaochun Y, Yongnian D, Bin Y, Wenhui M, Watanabe T (2007) J Wuhan Univ Technol-Mater Sci 22:307–310
Wakihara M, Ikuta H, Uchimoto Y (2002) Ionics 8:329–338
Shpak AY, Andriyko YO, Vlasenko NY, Andriiko AA (2010) Res Bull NTUU "KPI" 3:138–142
Cupid DM, Lehmann T, Berndt H, Seifert HJ (2013) J Mater Sci 48:3395–3403
Paulsen JM, Dahn JR (1999) Chem Mater 11:3065–3079
Andriiko AA, Shpak AYe, Andriyko YuO, Garcia Jose R, Khainakov SA, Vlasenko NY (2012) J Solid State Electrochem 16:1993-1998
Andriiko AA, Shpak AY, Vlasenko NY, Stepanenko NM (2008) Chem Metals and Alloys 1:283–287
Colby R (2013) Brown, McCalla E, Dahn JR. Solid State Ionics 253:234–238
Morgan KR, Collier S, Burns G, Ooi K (1994) J Chem Soc. Chem Commun 1719-1720
Verhoeven VWJ, de Schepper IM, Nachtegaal G, Kentgens APM, Kelder EM, Schoonman J, Mulder FM (2001) Phys Rev Lett 86:4314–4317
Paik Y, Grey CP, Johnson CS, Kim JS, Thackeray MM (2002) Chem Mater 14:5109–5115
Acknowledgments
SKKS and JD acknowledge the Région Pays de la Loire for the financial support (Convention No. 2007-11860).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shpak, A.Y., Kumara Swamy, S.K., Dittmer, J. et al. Formation of stable phases of the Li–Mn–Co oxide system at 800 °C under ambient oxygen pressure. J Solid State Electrochem 20, 87–94 (2016). https://doi.org/10.1007/s10008-015-3001-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3001-x