Abstract
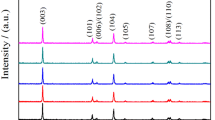
Decrystallization of cathode material and decomposition of electrolyte in LiNi1/3Co1/3Mn1/3O2-based cells are studied in this work. Surface morphology of the synthesized material is observed by scanning electron microscope (SEM). Initial charge–discharge capacities and cycling performance are measured in the voltage range of 2.75–4.30 V (vs. Li+/Li). The changes on crystal structure, surface elements of electrodes, and battery impedance are characterized by means of x-ray diffraction (XRD), energy-dispersive spectroscopy (EDS), and electrochemical impedance spectroscopy (EIS), respectively. The results show that the capacity fade is mainly caused by decrystallization of the cathode material and decomposition of electrolyte. During the cycling process, crystal structure is badly destroyed, degree of cation mixing increases, and amorphous phase forms, which will directly lead to the increase of irreversible capacity. Moreover, battery impedance will increase rapidly because the surface of electrode is covered and diffusion path of lithium ions is blocked by impurities generated from the decomposition of the electrolyte.






Similar content being viewed by others
References
Wu MS, Chiang PCJ (2007) Electrochim Acta 52:3719–3725
Ramadass P, Haran B, White R, Popov BN (2002) J Power Sources 112:606–613
Tarascon JM, Armand M (2001) Nature 414:359–367
Ahn D, Lee JG, Lee JS, Kim J, Cho J, Park B (2007) Curr Appl Phys 7:172–175
Park BC, Kim HB, Myung ST, Amine K, Belharouak I, Lee SM, Sun YK (2008) J Power Sources 178:826–831
Ning G, White RE, Popov BN (2006) Electrochim Acta 51:2012–2022
Ramadass P, Haran B, White R, Popov BN (2003) J Power Sources 123:230–240
Arora P, White RE (1998) J Electrochem Soc 145:3647–3667
Ramadass P, Haran B, White R, Popov BN (2002) J Power Sources 112:614–620
Zhang D, Haran BS, Durairajan A, White RE, Podrazhansky Y, Popov BN (2000) J Power Sources 91:122–129
Wang F, Zhang Y, Zou JZ, Liu WJ, Chen YP (2013) J Alloys Compd 558:172–178
Ohzuku T, Makimura Y (2001) Chem Lett 30:642–643
Sinha NN, Munichandraiah N (2009) ACS Appl Mater Interfaces 1:1241–1249
Käbitz S, Gerschler JB, Ecker M, Yurdagel Y, Emmermacher B, André D, Mitsch T, Sauer DU (2013) J Power Sources 239:572–583
Zheng H, Sun Q, Liu G, Song X, Battaglia VS (2012) J Power Sources 207:134–140
Kamel KB, Amdouni N, Ghany AA, Zaghib K, Mauger A, Gendron F, Julien CM (2008) Ionics 14:89–97
Liao PY, Duh JG, Sheen SR (2005) J Electrochem Soc 152:A1695–A1700
Ohzuku T, Ueda A, Nagayama M, Iwakoshi Y, Komori H (1993) Electrochim Acta 38:1159–1167
Reimers JN, Rossen E, Jones CD, Dahn JR (1993) Solid State Ion 61:335–344
Yang K, Fan LZ, Guo J, Qu X (2012) Electrochim Acta 63:363–368
Huang ZL, Gao J, He XM, Li JJ, Jiang CY (2012) J Power Sources 202:284–290
Zhang S (2007) Electrochim Acta 52:7337–7342
Aurbach D, Markovsky B, Levi MD, Schechter A, Moshkovich M, Cohen Y (1999) J Power Sources 81–82:95–111
Aurbach D, Gamolsky K, Markovsky B, Gofer Y, Schmidt M, Heider U (2002) Electrochim Acta 47:1423–1439
Liu H, Tan L (2011) Mater Chem Phys 129:729–732
He YB, Ning F, Yang QH, Song QS, Li B, Su F, Du H, Tang ZY, Kang F (2011) J Power Sources 196:10322–10327
Chen M, Du C, Song B, Xiong K, Yin G, Zuo P, Cheng X (2013) J Power Sources 223:100–106
Ni J, Han Y, Gao L, Lu L (2013) Electrochem Commun 31:84–87
Chen G, Wang Z, Xia D (2008) Chem Mater 20:6951–6956
Padhi AK, Nanjundaswamy KS, Masquelier C, Okada S, Goodenough JB (1997) J Electrochem Soc 144:1609–1613
Liu H, Li C, Zhang HP, Fu LJ, Wu YP, Wu HQ (2006) J Power Sources 159:717–720
Acknowledgments
The authors gratefully acknowledge financial support by The National Basic Research Program of China (973 program no. 2013CB934700).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, Y., Chen, B. et al. Study on decrystallization of cathode material and decomposition of electrolyte in LiNi1/3Co1/3Mn1/3O2-based cells. J Solid State Electrochem 18, 1757–1762 (2014). https://doi.org/10.1007/s10008-014-2419-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2419-x