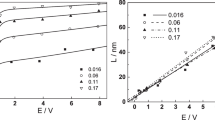
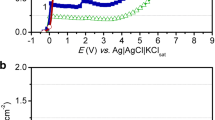
Abstract
Anodic oxidation has proven to be a promising route for the growth of self-ordering oxide nanotubes on Ti, the best results being obtained in ethylene glycol (EG)-based electrolytes with the addition of fluoride and small amounts of water. In the present paper, emphasis is put on the investigation of barrier film growth and dissolution on Ti in EG electrolytes with the addition of H2O (0.3–2.4 M) and NH4F (0.015–0.17 M) using electrochemical and surface analytical techniques. Steady-state current–potential curves and electrochemical impedance spectra as depending on potential (−0.1/5.0 V vs. AgCl/Ag), water and fluoride content have been registered. In addition, the chemical composition of the surface of the oxides obtained at 0.1 and 1.0 V has been estimated by X-ray photoelectron spectroscopy (XPS). XPS analysis revealed the presence of a non-stoichiometric oxide containing mainly Ti4+ and a certain amount of Ti3+, with a certain degree of hydroxylation. Estimates of the total thickness of the oxide from the XPS data using a dual layer model are also presented. A kinetic model of the process is advanced to quantitatively interpret the electrochemical and surface analytical results.









Similar content being viewed by others
References
Grimes CA, Varghese OK, Ranjan S (2008) Oxide semiconductors: nano-crystalline, tubular and porous systems. In Light, water, hydrogen. The solar generation of hydrogen by water photoelectrolysis. Springer, New York, chapter 5, pp 257–369
Grimes CA, Mor GK (2009) TiO2 nanotube arrays. Synthesis, properties, and applications. Springer, New York
Schmuki P (2009) Self-organized oxide nanotube layers on titanium and other transition metals. In: Schmuki P, Virtanen S (eds) Electrochemistry at the nanoscale. Springer, New York, chapter 12, pp 435–466
Bojinov M (2011) Nanoporous anodic oxides. In: Sattler K (ed) Handbook of nanophysics, Volume 5, Functional nanomaterials, chapter 14. Taylor and Francis, Boca Raton, pp 14–1–14–21
Prakasam HE, Shankar K, Paulose M, Grimes CA (2007) A new benchmark for TiO2 nanotube array growth by anodization. J Phys Chem C 111:7235–7241
Thompson GE, Wood GC (1981) Porous anodic film formation on aluminium. Nature 290:230–232
Parkhutik VP, Shershulsky J (1992) Theoretical modelling of porous oxide growth on aluminium. J Phys D Appl Phys 25:1258–1263
Skeldon P, Thompson GE, Garcia-Vergara SJ, Iglesias-Rubianes L, Blanco-Pinzon CE (2006) A Tracer study of porous anodic alumina. Electrochem Solid-State Lett 9:B47–B51
Houser JE, Hebert KR (2006) Modeling the potential distribution in porous anodic alumina films during steady-state growth. J Electrochem Soc 153:B566–B573
Houser JE, Hebert KR (2008) Stress-driven transport in ordered porous anodic films. Phys Status Solidi A 205:2396–2399
Houser JE, Hebert KR (2009) The role of viscous flow of oxide in the growth of self-ordered porous anodic alumina films. Nat Mater 8:415–420
Stanton LG, Golovin AA (2009) Effect of ion migration on the self-assembly of porous nanostructures in anodic oxides. Phys Rev B 79:035414
Sheintuch M, Smagina Y (2007) Nanopore formation dynamics during aluminum anodization. Physica D 226:95–105
Van Overmeere Q, Nysten B, Proost J (2009) In situ detection of porosity initiation during aluminum thin film anodizing. Appl Phys Lett 94:074103
Macak JM, Hildebrand H, Marten-Jahns U, Schmuki P (2008) Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J Electroanal Chem 621:254–266
Thébault F, Vuillemin B, Oltra R, Kunze J, Seyeux A, Schmuki P (2009) Modeling of growth and dissolution of nanotubular titania in fluoride-containing electrolytes. Electrochem Solid State Lett 12:C5–C9
Bhargava YV, Nguyen QA, Devine TM (2009) Initiation of organized nanopore/nanotube arrays in anodized titanium oxide I. Criterion for initiation. J Electrochem Soc 156:E55–E61
Nguyen QA, Bhargava YV, Devine TM (2009) Initiation of organized nanopore/nanotube arrays in anodized titanium oxide II. Nanopore size and spacing. J Electrochem Soc 156:E62–E68
Hebert KR, Albu SP, Paramasivam I, Schmuki P (2011) Morphological instability leading to formation of porous anodic oxide films. Nat Mater 11:162–166
Albu SP, Taccardi N, Paramasivam I, Hebert KR, Schmuki P (2012) Oxide growth efficiencies and self-organization of TiO2 nanotubes. J Electrochem Soc 159:H697–H703
Bojinov M, Cattarin S, Musiani M, Tribollet B (2003) Evidence of coupling between film growth and metal dissolution in passivation processes. Electrochim Acta 48:4107–4117
Karastoyanov V, Bojinov M (2008) Anodic oxidation of tungsten in sulphuric acid solution—influence of hydrofluoric acid addition. Mater Chem Phys 112:702–711
Karastoyanov V, Bojinov M (2009) Mechanism of anodic oxidation of tungsten in neutral sulphate-fluoride solutions. J Solid State Electrochem 13:309–321
Bojinov M, Karastoyanov V, Tzvetkov B (2010) Barrier layer growth and nanopore initiation during anodic oxidation of tungsten and niobium. ECS Trans 25:89–102
Betova I, Bojinov M, Karastoyanov V, Kinnunen P, Saario T (2010) Estimation of kinetic and transport parameters by quantitative evaluation of EIS and XPS data. Electrochim Acta 55:6163–6173
Frateur I, Cattarin S, Musiani M, Tribollet B (2000) Electrodissolution of Ti and p-Si in acidic fluoride media: formation ratio of oxide layers from electrochemical impedance spectroscopy. J Electroanal Chem 482:202–210
Acevedo-Pena P, Gonzalez I (2012) EIS characterization of the barrier layer formed over Ti during its potentiostatic anodization in 0.1 M HClO4/x mM HF (1 mM ≤ x ≤ 500 mM). J Electrochem Soc 159:C101–C108
Raja KS, Gandhi T, Misra M (2007) Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes. Electrochem Commun 9:1069–1076
Valota A, LeClere DJ, Skeldon P, Curioni M, Hashimoto T, Berger S, Kunze J, Schmuki P, Thompson GE (2009) Influence of water content on nanotubular anodic titania formed in fluoride/glycerol electrolytes. Electrochim Acta 54:4321–4327
Berger S, Kunze J, Schmuki P, Valota AT, LeClere DJ, Skeldon P, Thompson GE (2010) Influence of water content on the growth of anodic TiO2 nanotubes in fluoride-containing ethylene glycol electrolytes. J Electrochem Soc 157:C18–C23
Wei W, Berger S, Hauser C, Meyer K, Yang M, Schmuki P (2010) Transition of TiO2 nanotubes to nanopores for electrolytes with very low water contents. Electrochem Commun 12:1184–1186
Bojinov M, Betova I, Raicheff R (1996) Kinetics of formation and properties of a barrier oxide film on molybdenum. J Electroanal Chem 411:37–42
Bojinov M (1997) The ability of a surface charge approach to describe barrier film growth on tungsten in acidic solutions. Electrochim Acta 42:3489–3497
Bojinov M (1997) Modelling the formation and growth of anodic passive films on metals in concentrated acid solutions. J Solid State Electrochem 1:161–171
Biaggio SR, Rocha-Filho RC, Vilche JR, Varela FE, Gassa LM (1997) A study of thin anodic WO3 films by electrochemical impedance spectroscopy. Electrochim Acta 42:1751–1758
Cattarin S, Musiani M, Tribollet B (2002) Nb electrodissolution in acid fluoride medium steady-state and impedance investigations. J Electrochem Soc 149:B457–B464
Metikos-Hukovic M, Grubac Z (2003) The growth kinetics of thin anodic WO3 films investigated by electrochemical impedance spectroscopy. J Electroanal Chem 556:167–178
Sapra S, Li H, Wang Z, Suni II (2005) Voltammetry and impedance studies of Ta in aqueous HF. J Electrochem Soc 152:B193–B197
Kong DS (2010) Anion-incorporation model proposed for interpreting the interfacial physical origin of the faradaic pseudocapacitance observed on anodized valve metals; with anodized titanium in fluoride-containing perchloric acid as an example. Langmuir 26:4880–4891
Curioni M, Koroleva EV, Skeldon P, Thompson GE (2010) Flow modulated ionic migration during porous oxide growth on aluminium. Electrochim Acta 55:7044–7049
Giovanardi R, Fontanesi C, Dallabarba W (2011) Adsorption of organic compounds at the aluminium oxide/aqueous solution interface during the aluminium anodizing process. Electrochim Acta 56:3128–3138
Castro JAP, Quintero-Torres R, de Tacconi NR, Rajeshwar K, Chanmanee W (2011) Anodic growth of titania nanotube array on titanium substrate: a study by electrochemical impedance spectroscopy. J Electrochem Soc 158:D84–D90
Jonscher AK (1986) The physical origin of negative capacitance. J Chem Soc Faraday Trans 2(82):75–81
Göpel W, Anderson JA, Frankel D, Jaehnig M, Phillips K, Schäfer JA, Rocker G (1984) Surface defects of TiO2(110): a combined XPS, XAES and ELS study. Surf Sci 139:333–346
Antony RP, Mathews T, Dash S, Tyagi AK, Raj B (2012) X-ray photoelectron spectroscopic studies of anodically synthesized self aligned TiO2 nanotube arrays and the effect of electrochemical parameters on tube morphology. Mater Chem Phys 132:957–966
Yang B, Ng CK, Fung MK, Ling CC, Djurisic AB, Fung S (2011) Annealing study of titanium oxide nanotube arrays. Mater Chem Phys 130:1227–1231
Huang HH (2002) Effects of fluoride concentration and elastic tensile strain on the corrosion resistance of commercially pure titanium. Biomater 23:59–63
Habazaki H, Fushimi K, Shimizu K, Skeldon P, Thompson GE (2007) Fast migration of fluoride ions in growing anodic titanium oxide. Electrochem Commun 9:1222–1227
Berger S, Albu SP, Schmidt-Stein F, Hildebrand H, Schmuki P, Hammond JS, Paul DF, Reichlmaier S (2011) The origin for tubular growth of TiO2 nanotubes: A fluoride rich layer between tube-walls. Surf Sci 605:L57–L60
Macdonald DD (1992) The point defect model for the passive state. J Electrochem Soc 139:3434–3449
Acknowledgments
The financial support of the by the National Science Fund, Bulgarian Ministry of Education and Science, under contract DDVU-02-103 “Nanoporous anodic oxides as new generation of optically active and catalytic materials (NOXOAC, 2010–2013)” is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stancheva, M., Bojinov, M. Interfacial and bulk processes during oxide growth on titanium in ethylene glycol-based electrolytes. J Solid State Electrochem 17, 1271–1283 (2013). https://doi.org/10.1007/s10008-012-1990-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1990-2