Abstract
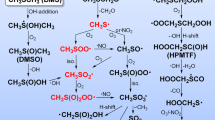
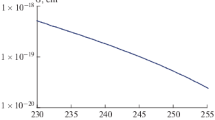
Electrode reactions of intermediates formed during capture of OH radicals by dimethylsulfoxide molecules were studied by laser photoemission in aqueous buffer solutions and pH range from acidic to basic. The results were compared with those obtained previously for electrochemical behaviour of methyl radicals generated via photoemission from CH3Cl. The essential similarity was found for parameters of irreversible one-electron transfer from/to these intermediates, i.e. the potentials E 1/2 on time-resolved voltammograms and rate constants at E = E 1/2. Hence, both active particles were concluded to be equivalent and corresponded to methyl radical. The primary product of OH radicals capture by DMSO molecules, i.e. adduct (CH3)2SO·(OH), was spontaneously decomposed to form ·CH3 with time as low as <2 × 10−5 s. A simultaneous increase of the reduction wave height was observed at pH transition from low basic to low acidic and at illumination times T m of an electrode with UV light if T m ≥ 90–300 ms. The increase exceeded considerably the one-electron reduction level. These features were presumably caused by the rather slow formation of organomercury intermediates as interaction products of the components of the system with a mercury electrode.




Similar content being viewed by others
References
Ludvik J, Pragst F, Volke J (1984) Electrochemical generation of triplet states: simplified estimation of triplet energies by electrogenerated chemiluminescence based on the anodic cleavage of dimeric dihydroheteroarenes. J Electroanal Chem 180(1–2):141
Ludvik J, Volke J, Pragst F (1986) Investigation of 2 radical intermediates in the anodic-oxidation of 1,4-dihydropyridines by electrochemiluminescence. J Electroanal Chem 215(1–2):179
Ludvik J (1994) Elektrochemicky generovaná luminescence (in Czech). Chem Listy 88(11):696
Wayner DDM, Parker VD (1993) Bond-energies in solution from electrode-potentials and thermochemical cycles—a simplified and general-approach. Acc Chem Res 26(5):287
Grampp G, Muresanu C, Landgraf S (2005) Solvent influence on the electrochemical reduction of photochemically generated cis-azobenzene. J Electroanal Chem 582(1–2):171
Krivenko AG, Benderskii VA (1990) Electrochemistry of short-lived intermediates. Russ Chem Rev 59(1):1
Benderskii VA, Benderskii AV (1995) Laser electrochemistry of intermediates. CRC, New York
Krivenko AG, Kotkin AS, Kurmaz VA (2005) Thermodynamic and kinetic characteristics of intermediates of electrode reactions: determination by direct and combined electrochemical methods. Russ J Electrochem 41(2):122
Krivenko AG, Kotkin AS, Kurmaz VA (2005) Thermodynamic and kinetic characteristics of intermediates of electrode reactions: a comparative investigation of a number of alkylaryl and alkyl halide radicals by the laser photoemission methods. Russ J Electrochem 41(2):137
Gründler P, Kirbs A, Dunsch L (2009) Modern thermoelectrochemistry. ChemPhysChem 10(11):1722
Lund H (1983) Practical problems in electrochemistry. In: Lund H, Baizer MM (eds) Organic electrochemistry. An introduction and a guide, 2nd edn. Marcel Dekker, New York, pp 161–233
Gritzner G (2011) Half a century of electrochemistry, mainly in non-aqueous electrolytes (a personal view). J Solid State Electrochem 15 (7/8) 1791
Buxton GV, Greenstock CL, Helman WPh, Ross AR (1988) Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (.OH/.O−) in aqueous-solution. J Phys Chem Ref Data 17(2):513
Hapiot Ph, Konovalov VV, Savéant J-M (1995) Application of laser pulse photoinjection of electrons from metal electrodes to the determination of reduction potentials of organic radicals in aprotic solvents. J Am Chem Soc 117(4):1428
Krivenko AG, Kotkin AS, Kurmaz VA (2002) Prospects for determination of thermodynamic and kinetic parameters of intermediates of electrode reactions by laser photoemission. Mendeleev Commun 12(1):11
Dixon WT, Norman ROC, Buley AJ (1964) Electron spin resonance studies of oxidation. 2. Aliphatic acids + substituted acids. J Chem Soc 3625
Woodward JR, Lin TS, Sakaguchi Y, Hayashi H (2000) Detection of transient intermediates in the photochemical reaction of hydrogen peroxide with dimethyl sulfoxide by time-resolved EPR techniques. J Phys Chem A 104(3):557
Veltwisch D, Janata E, Asmus K-D (1980) Primary processes in the reaction of OH·-radicals with sulphoxides. J Chem Soc Perkin Trans 2(1):146
Song JF, Chen ZP, Guo W, Wang FM (2003) In situ generation and detection of methyl radical by voltammetry. Chin Sci Bull 48(11):1093
Koulkes-Pujo AM, Moreau M, Sutton J (1981) Methane formation from the reactions of hydroxyl radicals and hydrogen-atoms with dimethylsulfoxide (DMSO). FEBS Lett 129(1):52
Herscu-Kluska R, Masarwa A, Saphier M, Cohen H, Meyerstein D (2008) Mechanism of the reaction of radicals with peroxides and dimethyl sulfoxide in aqueous solution. Chem Eur J 14(19):5880
Eberhardt MK, Colina R (1988) The reaction of OH radicals with dimethyl sulfoxide. A comparative study of Fenton’s reagent and the radiolysis of aqueous dimethyl sulfoxide solutions. J Org Chem 53(5):1071
Bertilsson B-M, Gustafsson B, Kühn I, Torssel K (1970) Generation of radicals from sulphoxides with Fentons reagent—new radical alkylation method. Acta Chem Scand 24(10):3590
Raju MR, Schillaci ME, Carpenter SG, Goodhead DT, Ward JF (1996) Radiobiology of ultrasoft X rays. V. Modification of cell inactivation by dimethyl sulfoxide. Radiat Res 145(5):563
Zou H, Tai C, Gu XX, Zhu RH, Guo QH (2002) A new simple and rapid electrochemical method for the determination of hydroxyl radical generated by Fenton reaction and its application. Anal Bioanal Chem 373(1–2):111
Scholz F, Gonzalez GLL, de Carvalho LM, Hilgemann M, Brainina KZ, Kahlert H, Jack RS, Minh DT (2007) Indirect electrochemical sensing of radicals and radical scavengers in biological matrices. Angew Chem Int Ed 46(42):8079
Hilgemann M, Scholz F, Kahlert H, de Carvalho LM, da Rosa MB, Lindequist U, Wurster M, do Nascimento PC, Bohrer D (2010) Electrochemical assay to quantify the hydroxyl radical scavenging activity of medicinal plant extracts. Electroanal 22(4):406
Zhu AW, Liu Y, Rui Q, Tian Y (2011) Selective and sensitive determination of hydroxyl radicals generated from living cells through an electrochemical impedance method. Chem Commun 47(14):4279
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109(12):6570
Toffel P, Henglein A (1977) Polarogram of the free hydrogen atom and of some simple organic radicals. Discuss Faraday Soc 63:124
Schiffrin DJ (1973) Application of the photo-electrochemical effect to the study of the electrochemical properties of radical: CO −2 · and ·CH3. Discuss Faraday Soc 56:75
Benderskii VA, Krivenko AG, Kurmaz VA, Simbirtseva GV (1988) Reactions of normal-alkyl radicals at the mercury-electrode. Sov Electrochem 24(1):141
Eberson L (1999) Problems and prospects of the concerted dissociative electron transfer mechanism. Acta Chem Scand 53(10):751
Ludvik J, Nygård B (1996) Electrochemistry of aromatic diselenides and ditellurides in aprotic media. Preceding formation of mercury-containing compounds. Electrochim Acta 41(10):1661
Ludvik J, Nygård B (1997) Electrochemistry and metal complex formation of organic diselenides. J Electroanal Chem 423(1–2):1
Kurmaz VA, Gultyai VP (2010) Electrode reactions and electroanalysis of organomercury compounds. Russ Chem Rev 79(4):307
Kurmaz VA, Ershler AB (2006) Acid-catalysed degradation of the organomercury intermediates of pentafluorophenylmercury bromide reduction near a mercury electrode. Mendeleev Commun 16(4):234
Rufman NM, Rotenberg ZA (1980) Special kinetic features of the photo-decomposition of organolead compounds at lead electrode surfaces. Sov Electrochem 16(3):309
Wang LM, Zhang JS (2002) Ab initio study of reaction of dimethyl sulfoxide (DMSO) with OH radical. Chem Phys Lett 356(5–6):490
Bartels DM, Lawler RG, Trifunac AD (1985) Electron T 1 measurements in short-lived free-radicals by dynamic polarization recovery. J Chem Phys 83(6):2686
Opstad CL, Melo TB, Sliwka HR, Partali V (2009) Formation of DMSO and DMF radicals with minute amounts of base. Tetrahedron 65(36):7616
Chaudhri SA, Göbl M, Freyholdt T, Asmus K-D (1984) A method to generate and study (CH3)2S+. radical cations. Reduction of Me2SO by H. atoms in aqueous HClO4 solutions. J Am Chem Soc 106(20):5988
Occhialini D, Kristensen JS, Daasbjerg K, Lund H (1992) Estimation of reduction and standard potentials of some allyl and substituted alkyl radicals. Acta Chem Scand 46(5):474
Lund H, Skov K, Pedersen SU, Lund T, Daasbjerg K (2000) On the determination and use of reduction potentials of short-lived radicals. A review. Collect Czech Chem Commun 65(6):829
Krivenko AG, Kotkin AS, Kurmaz VA (2002) Mechanism of electroreduction of intermediates with and without a proton donor. Electrochim Acta 47(24):3891
Acknowledgements
The authors are grateful to Prof. Alexander G. Krivenko for helpful discussion. This work is supported by the Russian Foundation for Basic Research (project 09-03-00598).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to the 70th birthday of Fritz Pragst.
Rights and permissions
About this article
Cite this article
Kurmaz, V.A., Kotkin, A.S. & Simbirtseva, G.V. Laser photoemission generation and electrochemical study of methyl radicals as secondary products of OH radicals capture by dimethyl sulfoxide molecules. J Solid State Electrochem 15, 2119–2126 (2011). https://doi.org/10.1007/s10008-011-1534-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1534-1