Abstract
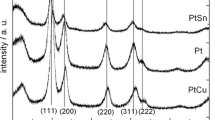
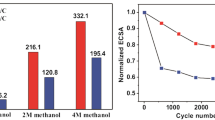
Pt nanocatalysts supported on glassy carbon (GC) were electrochemically deposited by cyclic voltammetry (CV) with different scanning potential ranges. The lower limit of potential was fixed at −0.25 V vs. saturated calomel electrode, whereas the upper limit of potential was adjusted to be 0.0, 0.20, 0.60, and 1.0 V. Scanning electron microscopy images showed that Pt microparticles are uniformly dispersed on the GC substrate and the agglomerated microparticles are composed of numerous nanoparticles. In addition, the catalytic capabilities of Pt/GCs for methanol electrooxidation were examined by CV, chronoamperometry, and electrochemical impedance spectroscopy in a solution of 0.5 M CH3OH and 0.5 M H2SO4. The results demonstrate that the catalytic activities and stabilities of Pt catalysts prepared by the potential ranges from −0.25 to both 0.60 and 1.0 V for methanol electrooxidation were higher than the others, which may be due to their higher electrochemical active surface area, lower charge transfer resistance, and more preferred Pt crystallographic orientation.











Similar content being viewed by others
References
Dyer CK (2002) J Power Sources 106:31. doi:10.1016/S0378-7753(01)01069-2
Dillon R, Srinivasan S, Aricò AS, Antonucci V (2004) J Power Sources 127:112. doi:10.1016/j.jpowsour.2003.09.032
Wasmus S, Kuver A (1999) J Electroanal Chem 461:14. doi:10.1016/S0022-0728(98)00197-1
Winter M, Brodd RJ (2004) Chem Rev 104:4245. doi:10.1021/cr020730k
Wang HW, Dong RX, Chang HY, Liu CL, Chen-Yang WC (2007) Mater Lett 61:830. doi:10.1016/j.matlet.2006.05.067
Chen WX, Lee JY, Liu ZL (2002) Chem Commun (Camb) 2588. doi:10.1039/b208600j
Zhao J, Chen WX, Zheng YF, Li X, Xu ZD (2006) J Mater Sci 41:5514. doi:10.1007/s10853-006-0276-4
Shim J, Joung KY, Ahn JH, Lee WM (2007) J Electrochem Soc 154:B165. doi:10.1149/1.2401032
Vanrheenen PR, Mckelvy MJ, Glaunsinger WS (1987) J Solid State Chem 67:151. doi:10.1016/0022-4596(87)90350-1
Koo IG, Lee MS, Shim JH, Ahn JH, Lee WM (2005) J Mater Chem 15:4125. doi:10.1039/b508420b
Whalen JJ, Weiland JD, Searson PC (2005) J Electrochem Soc 152:C738. doi:10.1149/1.2047407
Terzic S, Tripkovic D, Jovanovic VM, Tripkovic A, Kowal A (2007) J Serb Chem Soc 72:165. doi:10.2298/JSC0702165T
Rodrguez Nieto FJ, Pasquale MA, Cabrera CR, Arvia AJ (2006) Langmuir 22:10472. doi:10.1021/la0611716
Duarte MME, Pilla AS, Sieben JM, Mayer CE (2006) Electrochem Commun 8:159. doi:10.1016/j.elecom.2005.11.003
Custidiano E, Chialvo AC, Arvia AJ (1985) J Electroanal Chem 196:423. doi:10.1016/0022-0728(85)80038-3
Zubimendi JL, Vazquez L, Ocon P, Vara JM, Triaca WE, Salvarezza RC, Arvia AJ (1993) J Phys Chem 97:5095. doi:10.1021/j100121a041
Solla-Gullón J, Rodríguez P, Herrero E, Aldaz A, Feliu JM (2008) Phys Chem Chem Phys 10:1359. doi:10.1039/b709809j
Kinoshita K (1990) J Electrochem Soc 137:845. doi:10.1149/1.2086566
Stoyanova A, Naidenov V, Petrov K, Nikolov I, Vitanov T, Budevski E (1999) J Appl Electrochem 29:1197. doi:10.1023/A:1003482613323
Tran TD, Langer SH (1993) Anal Chem 65:1805. doi:10.1021/ac00061a027
Xu WL, Lu TH, Liu CP, Xing W (2005) J Phys Chem B 109:14325. doi:10.1021/jp051443y
Zhu J, Su Y, Cheng FJ, Chen J (2007) J Power Sources 166:331. doi:10.1016/j.jpowsour.2007.01.087
Manoharan R, Goodenough JB (1992) J Mater Chem 2:875. doi:10.1039/jm9920200875
Zhao GY, Xu CL, Guo DJ, Li H, Li HL (2006) J Power Sources 162:492. doi:10.1016/j.jpowsour.2006.06.082
Sugimoto W, Aoyama K, Kawaguchi T, Murakami Y, Takasu Y (2005) J Electroanal Chem 576:215. doi:10.1016/j.jelechem.2004.10.018
Wu G, Li L, Xu BQ (2004) Electrochim Acta 50:1. doi:10.1016/j.electacta.2004.07.006
Melnick RE, Palmore GTR (2001) J Phys Chem B 105:9449. doi:10.1021/jp003106p
Wang ZB, Yin GP, Shao YY, Yang BQ, Shi PF, Feng PX (2007) J Power Sources 165:9. doi:10.1016/j.jpowsour.2006.12.027
Acknowledgements
This work is supported by a grant from the Key Program of Basic Research of the Shanghai Committee of Science and Technology, China (grant no. 08JC1402000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, T., Jiang, R., Zhang, D. et al. AC impedance investigation of plating potentials on the catalytic activities of Pt nanocatalysts for methanol electrooxidation. J Solid State Electrochem 14, 101–107 (2010). https://doi.org/10.1007/s10008-009-0795-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-009-0795-4