Abstract
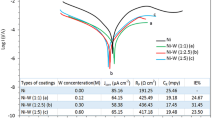
Ni–W alloys were electrodeposited onto copper foil from citrate solution. Coatings containing from 11 to 21 at.% W and having 7–52 μm in thickness were obtained. The structure of these alloys was analyzed by X-ray diffraction and by using electron and light microscopy techniques. Alloys with 11 and 15% W are composed of two phases: solid solution of W in fcc Ni and solid solution of Ni in bcc W. An increase in W content in the Ni–W alloys to ca. 18–19% of W resulted in the grain refinement and the transition to amorphous structure. The corrosion behavior of obtained Ni–W and unalloyed Ni coatings was studied in 0.5 M NaCl solution by means of electrochemical impedance spectroscopy, potentiodynamic polarization and light microscopy. Comparing to pure Ni, the obtained Ni–W coatings exhibited a clearly decreased corrosion resistance (in terms of corrosion current density and polarization or charge transfer resistance at the open circuit potential). Despite of the quite wide range of composition of the alloys under test, the related grain refinement, and the transition to the amorphous structure, no clear relation between the corrosion rate and W content was detected. This behavior can be a result of the interplay of the activating effect of grain refinement or preferential dissolution of W from one side and diffusion barrier action or inhibition provided by the surface film of W oxidation products from the other side. The differences observed in the corrosion resistance of Ni–W coatings are more related to their morphological imperfections arising from various deposition conditions than to the W content. Some samples showed a rather non-uniform nature of corrosion (pronounced attack along cracks). An inversion in the dissolution behavior of Ni–W and unalloyed Ni was observed with increasing anodic potential. Contrary to pure Ni, Ni–W coatings were resistant to pitting corrosion in NaCl solution.













Similar content being viewed by others
References
Sriraman KR, Ganesh Sundara Raman S, Seshadri SK (2007) Mater Sci Eng A 460–461:39 doi:10.1016/j.msea.2007.02.055
Eliaz N, Sridhar TM, Gileadi E (2005) Electrochim Acta 50:2893 doi:10.1016/j.electacta.2004.11.038
Pisarek M, Janik-Czachor M, Donten M (2008) Surf Coat Tech 202:1980 doi:10.1016/j.surfcoat.2007.08.047
Obradovic M, Stevanovic J, Despic A, Stevanovic R, Stoch J (2001) J Serb Chem Soc 66(11–12):899
Ren R, Wu YC, Shu X, Shi CW, Li Y, Zheng YC (2005) Trans Nonferous Met Soc China 15:198, in Chinese
Donten M, Stojek Z, Królikowski A, Płonska E (2007) 211th Meeting of the Electrochemical Society, Chicago, Abs 549
Lee SL, Lee YF, Chang MH, Lin JC (1999) Corros Prev Contr 46(3):71
Stepanova LI, Purovskaya OG (1998) Met Finish 96:50 doi:10.1016/S0026-0576(98)80871-4
Ke ST, Lee JL, Hou KH, Ger MD (2006) J Technol 21:75, in Chinese
Atanassov N, Gencheva K, Bratova M (1997) Plat Surf Finish 84:67
Zhu L, Zhong Q, Liu J (2000) Plat Surf Finish 87:74
Królikowski A (2007) Ochr p Kor (in Polish) 50(4):140
Yao S, Zhao S, Guo H, Kowaka M (1996) Corrosion 52(3):183
Yamasaki T, Schlossmacher P, Erlich K, Ogino Y (1998) Nanostruct Mater 10(3):375 doi:10.1016/S0965-9773(98)00078-6
Galikova Z, Chovancova M, Danielik V (2006) Chem Pap 60(5):353 doi:10.2478/s11696-006-0064-2
Wu Y, Chang D, Kim D, Kwon S (2003) Surf Coat Tech 162:269 doi:10.1016/S0257-8972(02)00699-0
Wu Y, Chang D, Kim D, Kwon S (2003) Surf Coat Tech 173:259 doi:10.1016/S0257-8972(03)00449-3
Yang FZ, Guo YF, Huang L, Xu SK, Zhou SM (2004) Chin J Chem 2:228
Nawarro-Flores E, Chong Z, Omanovic S (2005) J Mol Catal Chem 226:179 doi:10.1016/j.molcata.2004.10.029
Somekawa T, Nieh TG, Higashi K (2004) Scr Mater 50:1561
Nasu T, Sakurai M, Kamiyama T, Usuki T, Uemura O, Yamasaki T (2002) J Non-Cryst Solids 312–314:319 doi:10.1016/S0022-3093(02)01702-7
Schuh CA, Nieh TG, Iwasaki H (2003) Acta Mater 51:431 doi:10.1016/S1359-6454(02)00427-5
Giga A, Kimoto Y, Takigawa Y, Higashi K (2006) Scr Mater 55:143 doi:10.1016/j.scriptamat.2006.03.047
Sulitanu N, Brinza F (2003) J Optoelectronics. Adv Mater 5(2):421
Yamasaki T (2000) Mater Phys Mech 1:127
Itoh K, Wang F, Watanabe T (2001) Nippon Kinzoku Gakkaishi 65(11):1023 in Japanese
Donten M, Cesiulis H, Stojek Z (2000) Electrochim Acta 45:3389 doi:10.1016/S0013-4686(00)00437-0
Donten M, Stojek Z (1996) J Appl Electrochem 26:665 doi:10.1007/BF00253466
Cesiulis H, Baltutiene A, Donten M, Donten ML, Stojek Z (2002) J Solid State Electrochem 6:237 doi:10.1007/s100080100225
Wang H, Yao S, Matsumura S (2002) Surf Coat Tech 157:166 doi:10.1016/S0257-8972(02)00151-2
Moussa SO, Ibrahim MAM, Abd El Rehim SS (2006) J Appl Electrochem 36:333 doi:10.1007/s10800-005-9069-8
Zhu L, Younes O, Ashkenasy N, Schacham-Diamand Y, Gileadi E (2002) Appl Surf Sci 200(1–4):1 doi:10.1016/S0169-4332(02)00894-2
Zhang L, Macdonald DD (1998) Electrochim Acta 43(18):2661 doi:10.1016/S0013-4686(97)00268-5
Sakai Y, Shitanda I, Itagaki M, Watanabe K, Yasuda K, Saitou M (2007) 7th Intern Symp on Electrochemical Impedance Spectroscopy, Argeles-sur-Mer, Abstract 96
Magalhaes AAO, Margarit ICP, Mattos OR (1999) Electrochim Acta 44:4281 doi:10.1016/S0013-4686(99)00143-7
Magalhaes AAO, Margarit ICP, Mattos OR (2004) J Electroanal Chem 572(2):433 doi:10.1016/j.jelechem.2004.07.016
Hsu CS, Mansfeld F (2001) Corrosion 57(9):747
Jacobsen T, West K (1995) Electrochim Acta 40:255 doi:10.1016/0013-4686(94)E0192-3
Campestrini P, van Westing EPM, Hovestad A, de Wit JHW (2002) Electrochim Acta 47:1097 doi:10.1016/S0013-4686(01)00818-0
Campestrini P, Terryn H, Vereecken J, de Wit JHW (2004) J Electrochem Soc 151(6):B370 doi:10.1149/1.1736683
Lloyd AC, Noel JJ, McIntyre S, Shoesmith DW (2004) Electrochim Acta 49:3015 doi:10.1016/j.electacta.2004.01.061
Zhang YZ, Yao M (1999) Trans IMF 77(2):78
Author information
Authors and Affiliations
Corresponding author
Additional information
Contribution to the Fall Meeting of the European Materials Research Society, Symposium D: 9th International Symposium on Electrochemical/Chemical Reactivity of Metastable Materials, Warsaw, 17th–21st September, 2007.
Rights and permissions
About this article
Cite this article
Królikowski, A., Płońska, E., Ostrowski, A. et al. Effects of compositional and structural features on corrosion behavior of nickel–tungsten alloys. J Solid State Electrochem 13, 263–275 (2009). https://doi.org/10.1007/s10008-008-0712-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0712-2