Abstract
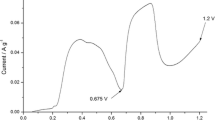
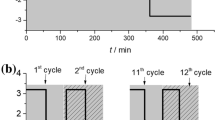
In the presented paper, we report on electrochemical oxidation of phenol occurring at exfoliated graphite (EG) in alkaline solution. The mechanism of the electrocatalytic reaction of phenol oxidation was modified on adding methanol to the phenol-containing electrolyte. Using the voltammetry method, the influence of methanol additive on cyclic behavior of EG electrode was examined. A particular attention has been paid to the first two cycles when an abrupt decrease in electrocatalytic activity of various electrode materials has been observed. The results obtained showed that in the presence of methanol EG, electrode preserves its electrocatalytic activity for a longer time of phenol oxidation. In the absence of methanol in a phenol/KOH electrolyte, the charge of phenol oxidation peaks decreases sharply on cycling, whereas in the presence of methanol, the observed drop is considerably inhibited. The anodic charge attained for the 15th cycle of phenol oxidation in methanol-admixed electrolyte is the same as that for the third cycle recorded in methanol-free electrolyte. The thermogravimetric analysis (TG), Fourier-transformed infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS) data showed that an improved electrocatalytic activity of EG can be accounted for by new chemical composition of oligomer film built on the EG surface with the participation of methanol and/or the products of its oxidation.








Similar content being viewed by others
References
Ayranci E, Conway BE (2001) J Electroanal Chem 513:100
Santos A, Yustos P, Durbán B, García-Ochoa F (2001) Catal Today 66:511
Renzi C, Guillard C, Herrmann J-M, Pichat P, Baldi G (1997) Chemosphere 35:819
Sá CSA, Boaventura RAR (2001) Biochem Eng J 9:211
Lapuente R, Cases F, Garcés P, Morallón E, Vázquez JL (1998) J Electroanal Chem 451:163
Gonçalves D, Faria RC, Yonashiro M, Bulhões LOS (2000) J Electroanal Chem 487:90
Eerskis Z, Jusys Z (2002) J Appl Electrochem 32:755
Kuramitz H, Nakata Y, Kawasaki M, Tanaka S (2001) Chemosphere 45:37
Ureta-Zañartu MS, Bustos P, Berrios C, Diez MC, Mora ML, Gutiérrez C (2002) Electrochim Acta 47:2399
Polcaro AM, Palmas S, Renoldi F, Mascia M (2000) Electrochim Acta 46:389
Johnson DC, Feng J, Houk LL (2000) Electrochim Acta 46:323
Kuramitz H, Saitoh J, Hattori T, Tanaka S (2002) Water Res 36:3323
Andreescu S, Andreescu D, Sadik OA (2003) Electrochem Commun 5:681
Zanta CLPS, Michaud P-A, Comninellis C, Andrade AR, Boodts JFC (2002) J Appl Electrochem 33:1211
Iniesta J, González-García J, Expósito E, Montiel V, Aldaz A (2001) Water Res 35:3291
Feng YJ, Li XY (2003) Water Res 37:2399
Cañizares P, García-Gómez J, Sáez C, Rodrigo MA (2003) J Appl Electrochem Part I 33:917
Skowroński JM, Krawczyk P (2000) Proc 51th Annual Meeting of International Society of Electrochemistry Warszawa (extended abstracts), p 218
Skowroński JM, Krawczyk P (2003) Eurocarbon (extended abstracts), Oviedo
Skowroński JM, Krawczyk P (2004) J Solid State Electrochem 8:242
Glarum SH, Marshal JH, Hellman MY, Taylor GN (1987) J Electrochem Soc 134:81
Gottrell M, Kirk DW (1992) J Electrochem Soc 139:2736
Gottrell M, Kirk DW (1993) J Electrochem Soc 140:903
Ežerskis Z, Jusys Z (2001) J Appl Electrochem Part I 31:1117
Zhang H (2002) Chem Eng J 85:81
Poon M, McCreery RL (1986) Anal Chem 58:2745
Skowroński JM, Jurewicz K (1991) Synth Met 40:161
Orozco G, Pérez MC, Rincón A, Gutiérrez C (2000) J Electroanal Chem 495:71
Zawadzki J, Azambre B, Heintz O, Krztoñ A, Weber J (2000) Carbon 38:509
Ramesh P, Sampath S (2001) Analyst 126:1872
Weng W, Chen G, Wu D, Lin Z, Yan W (2003) Synth Met 139:221
Biniak S, Szamański G, Siedlewski J, Świątkowski A (1997) Carbon 35:1799
László K, Tombácz E, Josepovitz K (2001) Carbon 39:1217
Darmstadt H, Roy C, Kaliaguine S, Choi SJ, Ryoo R (2002) Carbon 40:2673
Świątkowski A, Pakula M, Biniak S, Walczyk M (2004) Carbon 42:3057
Okpalugo TIT, Papakonstantinou P, Murphy H, McLaughlin, Brown NMD (2005) Carbon 43:153
Su F, Lv L, Hui TM, Zhao XS (2005) Carbon 43:1156
Harikumar KR, Rao CNR (1997) Catal Letters 47:265
Edmundts A, Pirug G, Werner J, Bonzel HP (1998) Surf Sci 410:L727
Kulkarni GU, Rao CNR (2003) Top Catal 22:183
Werner H, Herein D, Schultz G, Wild U, Schlögl (1997) Catal Letters 49:109
Bukhtiyarov VI, Prosvirin IP, Tikhomirov EP, Kaichev VV, Sorokin AM, Evstigneev VV (2003) React Kinet Catal Lett 79:181
Prosvirin IP, Tikhomirov EP, Sorokin AM, Kaichev VV, Bukhtiyarov VI (2003) Kinet Catal 44:724
Ramesh P, Bhagyalakshmi S, Sampath S (2004) J Colloid Interface Sci 274:95
Blyth RIR, Buqa H, Netzer FP, Ramey MG, Besenhard JO, Golob P, Winter M (2000) Appl Surf Sci 167:99
Acknowledgement
This work was financially supported by the grant DS 31-084/05.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skowroński, J.M., Krawczyk, P. Improved electrooxidation of phenol at exfoliated graphite electrodes. J Solid State Electrochem 11, 223–230 (2007). https://doi.org/10.1007/s10008-005-0092-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-005-0092-9