Abstract
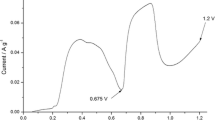
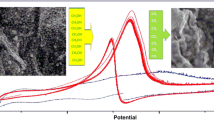
The present paper reports on exfoliated graphite (EG) used for the cyclic electrochemical process of phenol oxidation in alkaline solution. It is shown that the electrochemical activity of anode-produced EG decreases considerably in the second cycle due to the deposition of an oligomer film, composed of the products of phenol oxidation, on the EG surface. Thermal treatment of the inactive graphite anode in air at 500 °C provided a regenerated material of activity three times higher for the first cycle and 2.6 times higher for three cycles as compared to the original anode. The reason for such a behavior is assigned to a carbon film formed on the EG surface during the carbonization/oxidation processes involving the products of phenol oxidation. Comparative studies showed that electroactivity of the original EG can also be enhanced if before the process of phenol oxidation the original EG is activated by heat treatment. Unfortunately, the electrochemical activity of the product of such a treatment is higher only for the first cycle of phenol oxidation and drops dramatically in the following cycles.







Similar content being viewed by others
References
Skowroński JM (1988) Carbon 26:613
Bourelle E, Douglade J, Metrot A (1994) Mol Cryst Liq Cryst 244:227
Bourelle E, Claude-Montigny B, Metrot A (1998) Mol Cryst Liq Cryst 310:321
Skowroński JM (1988) J Mater Sci 23:2243
Chung DDL (1987) J Mater Sci 22:4190
Celzard A, McRae E, Marache JF, Furdin G, Dufort M, Deleuze C (1996) J Phys Chem Solids 57:715
Toyoda M, Moriya K, Lizawa J, Konno H, Inagaki M (2000) Desalination 128:205
Inagaki M, Konno H, Toyoda M, Moriya K, Kitara T (2000) Desalination 12:213
Inagaki M, Shibata K, Setou S, Toyoda M, Lizawa J (2000) Desalination 128:219
Tryba B, Kaleńczuk RJ, Kang F, Inagaki M, Morawski AW (2000) Mol Cryst Liq Cyst 340:113
Toyoda M, Inagaki M (2000) Carbon 38:199
Gottrell M, Kirk DW (1992) J Electrochem Soc 139:2736
Gottrell M, Kirk DW (1993) J Electrochem Soc 140:903
Lapuente R, Cases F, Garcés P, Morallón E, Vázquez JL (1998) J Electroanal Chem 451:163
Iotov PI, Kalcheva SV (1998) J Electroanal Chem 442:19
Boudenne JL, Cerclier O, Galéa J, Van der Vlist E (1996) Appl Catal A: General 143:85
Boudenne JL, Cerclier O, Galéa J, Van der Vlist E (1998) J Electrochem Soc 145:2763
Kuramitz H, Nakata Y, Kawasaki M, Tanaka S (2001) Chemosphere 45:37
Ureta-Zañartu MS, Bustos P, Berrios C, Diez MC, Mora ML, Gutiérrez C (2002) Electrochim Acta 47:2399
Salvador F, Sánchez Jiménez C (1996) Carbon 34:511
Chiang PC, Chung EE, Wu JS (1997) Water Sci Technol 35:279
Matatov-Meytal Yu, Sheintuch M, Shiter GE, Grader GS (1997) Carbon 35:1527
Salvador F, Sánchez Jiménez C (1999) Carbon 37:577
Sheintuch M, Matatov-Meytal YI (1999) Catal Today 53:73
San Miguel G, Lambert SD, Graham NJD (2001) Water Res 35:2740
Zhang H (2002) Chem Eng J 85:81
Shter GC, Shindler Yu, Matatov-Meytal Yu, Grader GS, Sheintuch M (2002) Carbon 40:2547
Crittenden JC, Suri RPS, Perram DL, Hand DW (1997) Water Res 31:411
Skowroński JM, Krawczyk P (2000) Proc 51st Annual Meeting of International Society of Electrochemistry, Warszawa, Extended Abstracts, p 218
Skowroński JM, Jurewicz K (1991) Synth Met 40:161
Acknowledgements
This work was financially supported from the grant TB 31-048/03 DS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Harry B. Mark, Jr. (28 February 1934–3 March 2003)
Contribution to the 3rd Baltic Conference on Electrochemistry, Gdańsk-Sobieszewo, 23–26 April 2003.
Rights and permissions
About this article
Cite this article
Skowroński, J.M., Krawczyk, P. Electrooxidation of phenol at exfoliated graphite electrode in alkaline solution. J Solid State Electrochem 8, 442–447 (2004). https://doi.org/10.1007/s10008-003-0483-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-003-0483-8