Abstract
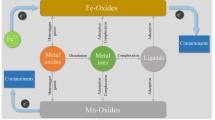
The presence of ligands in natural and artificial environments is increasing. Science majors need exposure to experiments that deal with ligands and complexes in the environment. Two such experiments are presented here: The dissolution of a nonsoluble polluting gas (NO) by complex formation with [Iron(II)EDTA] and the decomposition of [Iron(III)EDTA] by exposure to light. The experiments involve the preparation of Iron-EDTA complexes, which are then used to demonstrate the positive and negative effects of ligands in the environment. These experiments are appropriate for applied inorganic chemistry or environmental chemistry laboratories. The first one takes approximately three hours to complete, and the second takes less than two hours.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ibanez, J.G., Miranda-Treviño, J.C., Topete-Pastor, J. et al. Metal Complexes and the Environment: Microscale Experiments with Iron-EDTA Chelates. Chem. Educator 5, 226–230 (2000). https://doi.org/10.1007/s00897000414a
Issue Date:
DOI: https://doi.org/10.1007/s00897000414a