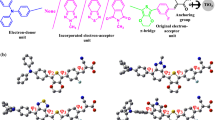
Abstract
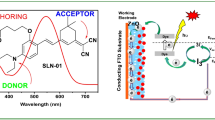
New dyes were developed and produced utilizing distinct electron donors (phenothiazine and dibenzofuran), a π-spacer, and an electron acceptor of cyanoacetohydrazide, and their structures were studied using FT-IR and NMR spectroscopy. Following the synthesis of dye molecules, the photophysical and photovoltaic characteristics were investigated using experimental and theoretical methods. The photosensitizers have been exposed to electrochemical and optical property experiments in order to study their absorption performance and also molecular orbital energies. The monochromatic optical conversion efficiency of (Z)-N-((5-(10H-phenothiazin-2-yl)furan-2-yl)methylene)-2-cyanoacetohydrazide (PFCH) was found higher than that of (Z)-2-cyano-N′-((5-(dibenzo[b,d]furan-4-yl)furan-2-yl)methylene)acetohydrazide (BFCH), with IPCEs of 58 and 64% for BFCH and PFCH, respectively. According to the photosensitizer molecular energy level diagram, the studied dye molecules have strong thermodynamically advantageous ground and excited-state oxidation potentials for electron injection into the conduction band of titanium oxide. It was observed that the ability to attract electrons correlated favorably with molecular orbital energy. While density functional theory calculations were used to examine molecule geometries, vertical electronic excitations, and frontier molecular orbitals, experimental and computed results were consistent. Natural bond orbital and nonlinear optical properties were also calculated and discussed.










Similar content being viewed by others
Data availability
The Supplementary Information Files include additional materials.
Code availability
No new codes have been created. Existing codes were utilized and quoted correctly.
References
Yella A, Lee HW, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MK, Diau EWD, Yeh CY, Zakeeruddin SM, Grätzel M (2011) Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334:629–634
Hemavathi B, Jayadev V, Praveen RC, Pai RK, Narayanan Unni KN, Ahipa TN, Soman S, GeethaBalakrishna R (2019) Variation of the donor and acceptor in D-A–π–A based cyanopyridine dyes and its effect on dye sensitized solar cells. New J Chem 43:15673–15680
SaravanaKumaran T, Prakasam A, Anbarasan PM, Vennila P, Venkatesh G, ParveenBanu S, Sheena Mary Y (2021) New phenoxazine-based organic dyes with various acceptors for dye-sensitized solar cells: synthesis, characterization, DSSCs fabrications and DFT study. J Comput Biophys Chem 20:1–12
Mathew S, Yella A, Gao P, Humphry-Baker R, Curchod BF, Ashari-Astani N, Tavernelli I, Rothlisberger U, Nazeeruddin MK, Grätzel M (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6:242–247
Yella A, Mai CL, Zakeeruddin CM, Chang SN, Hsieh CH, Yeh CY, Grätzel M (2014) Angew Chem 53:3017–3021
Amogne NY, Ayele DW, Tsigie YA (2020) Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater Renew Sustain Energy 9:23
Zhang X, Xu Y, Giordano F, Schreier M, Pellet N, Hu Y, Yi C, Robertson N, Hua J, Zakeeruddin SM, Tian H, Grätzel M (2016) J Am Chem Soc 138:10742–10745
Iqbal Z, Wu WQ, Huang ZS, Wang L, Kuang DB, Meier H, Cao D (2016) Trilateral π-conjugation extensions of phenothiazine based dyes enhance the photovoltaic performance of the dye-sensitized solar cells. Dyes Pigm 124:63–71
Koumura N, Wang Z-S, Mori S, Miyashita M, Suzuki E, Hara K (2008) Alkyl-functionalized organic dyes for efficient molecular photovoltaic. J Am Chem Soc 128:14256–21425
Justin Thomas KR, Kapoor N, Lee C-P, Ho K-C (2012) Organic dyes containing pyrenylamine-based cascade donor systems with different aromatic π linkers for dye-sensitized solar cells: optical, electrochemical, and device characteristics. Chem Asian J 7(4):738–750
Lee JK, Yang M (2011) Review progress in light harvesting and charge injection of dye sensitized solar cells. Mater Sci Eng B 176:1142–1160
Liu B, Zhu W, Zhang Q, Wu W, Xu M, Ning Z, Xie Y, Tian H (2009) Conveniently synthesized isophorone dyes for high efficiency dye-sensitized solar cells: tuning photovoltaic performance by structural modification of donor group in donor-π-acceptor system. Chem Commun 2:1766–1768
Horiuchi T, Miura H, Sumioka K, Uchida S (2004) High efficiency of dye-sensitized solar cells based on metal-free indoline dyes. J Am Chem Soc 126:12218–12219
Revoju S, Biswas S, Eliasson B, Sharma GD (2018) Asymmetric triphenylamine–phenothiazine based small molecules with varying terminal acceptors for solution processed bulk-heterojunction organic solar cells. Phys Chem Chem Phys 20:6390–6640
Slodek A, Zych D, Szafraniec-Gorol G, Gnida P, Vasylieva M, Schab-Balcerzak E (2020) Investigations of new phenothiazine-based compounds for dye-sensitized solar cells with theoretical insight. Materials 13(10):2292
Bourass M, Benjelloun AT, Benzakour M, Mcharfi M, Jhilal F, Serein-Spirau F, Sotiropoulos JM, Bouachrine M (2017) DFT/TD-DFT characterization of conjugational electronic structures and spectral properties of materials based on thieno[3,2-b][1]benzothiophene for organic photovoltaic and solar cell applications. J Saudi Chem Soc 21:563–574
Khayer K, Haque T (2020) Density functional theory calculation on the structural, electronic, and optical properties of fluorene-based azo compounds. ACS Omega 5:4507–4531
Slodek A, Zych D, Golba S, Zimosz S, Gnida P, Schab-Balcerzak E (2019) Impact of the donor structure in new D–π–A systems based on indolo[3,2,1-jk]carbazoles on their thermal, electrochemical, optoelectronic and luminescence properties. J Mater Chem C 7:5830–5840
Belić J, van Beek B, Menzel JP, Buda F, Visscher L (2020) Systematic computational design and optimization of light absorbing dyes. J Phys Chem A 124:6380–6388
Abdullah G, Al-Sehemi, Shuhrah Ali S, Allami AbulKalam (2020) Design and synthesis of organic dyes with various donor groups: promising dyes for dye-sensitized solar cells. Bull Mater Sci 43:224
Park JM, Jung CY, Wang Y, Choi HD, Park SJ, Ou P, Jang WD, Jaung JY (2019) Effect of additional phenothiazine donor and thiophene π-bridge on photovoltaic performance of quinoxaline cored photosensitizers. Dyes Pigms. 170:107568
Huang ZS, Meier H, Cao D (2016) Phenothiazine-based dyes for efficient dye-sensitized solar cells. J Mater Chem C 4:2404–2426
Buene AF, Uggerud N, Economopoulos SP, Gautun OR, Hoff BH (2018) Dyes Pigms 151:263–271
Ahmad S, Guillen E, Kavan L, Grätzel M, Nazeeruddin MK (2013) Metal free sensitizer and catalyst for dye sensitized solar cells. Energy Environ Sci 6:3439–3466
Zhou H, Ji JM, Kang SH, Kim MS, Lee HS, Kim CH, Kim HK (2019) Molecular design and synthesis of D–π–A structured porphyrin dyes with various acceptor units for dye-sensitized solar cells. J Mater Chem C 7:2843–2852
Velu S, Muniyasamy H, Ayyanar S, Maniarasu S, Veerappan G, Sepperumal M (2019) synthesis of organic sensitizers containing carbazole and triphenylamine π-bridged moiety for dye-sensitized solar cells. J Iran Chem Soc 16:1923–1937
Kim SH, Kim HW, Sakong C, Namgoong J, Park SW, Ko MK, Hyuk Lee C, Lee WI, Kim JP (2011) Effect of five-membered heteroaromatic linkers to the performance of phenothiazine-based dye-sensitized solar cells. Org Lett 13:5784–5787
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Asegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Ioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, AlLaham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 03, Revision E.01. Gaussian Inc., Pittsburgh, B.A. 2000. Walliford CT, 121:150-166
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of Hartree—Fock, Moller—Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Phys Chem 100:16502–16513
Islam N, Kaya S (2018) Conceptual density functional theory and its application in the chemical domain. CRC Press
Koopmans T (1934) über Die Zuordnung Von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica 1:104–113
Pearson RG (1999) Maximum chemical and physical hardness. J Chem Educ 76:267
Gazquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A 111:1966–1970
Palanisamy SP, Maheswaran G, GeethaSelvarani A, Kamal C, Venkatesh G (2018) Ricinus communis – a green extract for the improvement of anti-corrosion and mechanical properties of reinforcing steel in concrete in chloride media. J Build Eng 19:376–383
SaravanaKumaran T, Prakasam A, Venkatesh G, Kamal C, Sheena Mary Y, ParveenBanu S, Vennila P, Shyma Mary Y (2020) Synthesis, spectral characterizations, molecular geometries and electronic properties of phenothiazine based organic dyes for dye-sensitized solar cells. Z Phys Chem. https://doi.org/10.1515/zpch-2020-1732
Zanjanchi F, Beheshtian J (2018) Natural pigments in dye-sensitized solar cell (DSSC): A DFT-TDDFT study. J Iran Chem Soc 16:795–805. https://doi.org/10.1007/s13738-018-1561-2
SaravanaKumaran T, Prakasam A, Vennila P, ParveenBanu S, Venkatesh G (2021) New carbazole-based organic dyes with various acceptors for dye-sensitized solar cells: synthesis, characterization, DSSCs fabrications and DFT study. Asian J Chem 33:1541–1550
Harikrishnan M, Sadhasivam V, Siva A, Anandan S, Subbiah V, Murugesan S (2019) Energy level tuning of novel star-shaped D−π–D–A-based metal-free organic dyes for solar cell application. J Phys Chem C 123:21959–21968
Sun C, Li Y, Qi D, Li H (2016) Optical and electrical properties of purpurin and alizarin complexone as sensitizers for dye-sensitized solar cells. J Mater Sci: Mater Electron 27:8027–8039
Periyasamy K, Sakthivel P, Vennila P, Anbarasan PM, Venkatesh G, Sheena Mary Y (2021) Novel D-π-A phenothiazine and dibenzofuran organic dyes with simple structures for efficient dye-sensitized solar cells. J Photochem Photobiol A 413:113269
Batzill M (2011) Fundamental aspects of surface engineering of transition metal oxide photocatalysts. Energy Environm Sci 4:3275–3286
Pan J, Liu G, Lu GQ, Cheng HM (2011) On the true photoreactivity order of 001}, {010 and 101 facets of anatase TiO2 crystals. Angew Chem Int Ed 50:2133–2137
Zhang We Li, Yang Xichuan, Wang Weihan, Gurzadyan Gagik G, Li Jiajia, Li Xiaoxin, An Jincheng, Ze Yu, Wang Haoxin, Cai Bin, Hagfeldt Anders, Sun Licheng (2019) 13.6% efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state. ACS Energy Lett 4:943–95
Zeng K, Chen Y, Zhu W-H, Tian He, Xie Y (2020) Efficient solar cells based on concerted companion dyes containing two complementary components: an alternative approach for cosensitization. J Am Chem Soc 142:5154–5161
Ji J-M, Zhou H, Kim HK (2018) Rational design criteria for D–π–A structured organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells. J Mater Chem A 6:14518–14545
Athira M. John, Renjith Thomas, Sreeja P. Balakrishnan, Nabil Al-Zaqri, Ali Alsalme, Ismail Warad. Diazo-pyrazole analogues as photosensitizers in dye sensitised solar cells: tuning for a better photovoltaic efficiency using a new modelling strategy using experimental and computational data. Zeitschrift für Physikalische Chemie. https://doi.org/10.1515/zpch-2020-1722
Venkatesh G, Kamal C, Vennila P, Govindaraju M, Sheena Mary Y, Armakovic S, Armakovic SJ, Kaya S, YohannanPanicker C (2018) Molecular dynamic simulations, ALIE surface, Fukui functions geometrical, molecular docking and vibrational spectra studies of tetra chloro p and m-xylene. J Mol Struct 1171:253–267
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539
Saha R, Pan S, Chattaraj PK (2016) Molecules statistical significance of the maximum hardness principle applied to some selected chemical reactions. Molecules 21:1477
Ghanty TK, Ghosh SK (2000) Molecular hardness, polarizability and valency variation of formamide and thioformamide on internal rotation: a density functional study. J Phys Chem A 104:2975–2979
Chattaraj PK, Sarkar U, Roy DR (2006) Electrophilicity index. Chem Rev 106:2065–2091
Chamorro E, Chattaraj PK, Fuentealba P (2003) Variation of the electrophilicity index along the reaction path. J Phys Chem A 107:7068–7072
von Szentpály László, Kaya Savaş, Karakuş Nihat (2020) Why and when is electrophilicity minimized? New theorems and guiding rules. Am J Phys Chem 124:10897–10908
Vennila P, Govindaraju M, Venkatesh G, Kamal C (2016) Molecular structure, vibrational spectral assignments (FT-IR and FT-RAMAN), NMR, NBO, HOMO-LUMO and NLO properties of O-methoxybenzaldehyde based on DFT calculations. J Mol Struct 1111:151
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev in Phys Chem 35:399
Venkatesh G, Govindaraju M, Kamal C, Vennila P, Kaya S (2017) Structural, electronic and optical properties of 2,5 dichloro-p-xylene: experimental and theoretical calculations using DFT method. RSC Adv 7:1401
Acknowledgements
The authors would like to express their gratitude to Gandhigram Rural University and SAIF Chennai for providing spectral and electrochemical resources.
Author information
Authors and Affiliations
Contributions
K. Periyasamy: experimental, DFT studies, and manuscript writing.
P. Sakthivel: supervision, manuscript preparation, and data analysis.
G. Venkatesh: manuscript draft correction and method development and standardization.
P.M. Anbarasan: software and electrochemical data analysis.
P. Vennila: validation curation of data and method development.
Y. Sheena Mary: conceiving of the problem.
S. Kaya: FMO calculation.
Sultan Erkan: manuscript draft correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Periyasamy, K., Sakthivel, P., Venkatesh, G. et al. Synthesis, photophysical, electrochemical, and DFT examinations of two new organic dye molecules based on phenothiazine and dibenzofuran. J Mol Model 28, 34 (2022). https://doi.org/10.1007/s00894-022-05026-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05026-w