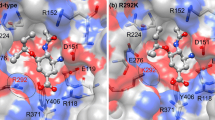
Abstract
The influenza virus is an important respiratory pathogen that causes many incidences of diseases and even death each year. One of the primary factors of this virus is the Neuraminidase surface protein, which causes the virus to leave the host cell and spread to new target cells. The main antiviral medication for influenza is designed as a protein inhibitor ligand that prevents further spread of the disease, and eventually relieves the emerged symptoms. The effectiveness of such inhibitory drugs is highly associated with their binding affinity. In this paper, the binding affinity of an herbal ligand of Capsaicin bound to Neuraminidase of the influenza virus is investigated using steered molecular dynamics (SMD) simulation. Since mutations of the virus directly impact the binding affinity of the inhibitory drugs, different mutations were generated by using Mutagenesis module. The rapid spread of infection during the avian influenza A/H5N1 epidemic has raised concerns about far more dangerous consequences if the virus becomes resistant to current drugs. Currently, oseltamivir (Tamiflu), zanamivir (Relenza), pramivir (Rapivab), and laninamivir (Inavir) are increasingly used to treat the flu. However, with the rapid evolution of the virus, some drug-resistant strains are emerging. Therefore, it is very important to seek alternative therapies and identify the roots of drug resistance. Obtained results demonstrated a reduced binding affinity for the applied mutations. This reduction in binding affinity will cause the virus mutation to become resistant to the drug, which will spread the disease and make it more difficult to treat. From a molecular prospect, this decrease in binding affinity is due to the loss of a number of effective bonds between the ligand and the receptor, which occurs with mutations of the wild-type (WT) species. The results of the present study can be used in the rational design of novel drugs that are compatible with specific mutations.





Similar content being viewed by others
Data Availability
N/A.
Code availability
N/A.
References
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Viruses: structure, function, and uses. In: Mol Cell Biol 4th edition. WH Freeman; 2000.
Abed Y, Baz M, Boivin G (2006) Impact of neuraminidase mutations conferring influenza resistance to neuraminidase inhibitors in the N1 and N2 genetic backgrounds. Antivir Ther 11(8):971
Daniels R, Nicoll LH. Contemporary medical-surgical nursing. Thomson Delmar Learning,; 2012.
Schnitzler P, Schön K, Reichling J (2001) Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 56(4):343–347
McKimm-Breschkin JL (2013) Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respi Viruses 7:25–36
Fujita J. Influenza: advances in diagnosis and management. Springer Nature; 2020.
Ikematsu H, Kawai N (2011) Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza. Expert Rev Anti Infect Ther 9(10):851–857
Liu Z, Zhao J, Li W, Shen L, Huang S, Tang J et al (2016) Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci Rep 6(1):1–9
Tam NM, Nguyen MT, Ngo ST (2017) Evaluation of the absolute affinity of neuraminidase inhibitor using steered molecular dynamics simulations. J Mol Graph Model 77:137–142
Selvaraj GF, Piramanayagam S, Devadasan V, Hassan S, Krishnasamy K, Srinivasan S. Computational analysis of drug like candidates against Neuraminidase of Human Influenza A virus subtypes. Informatics Med Unlocked. 2020;18:100284.
Wang NX, Zheng JJ (2009) Computational studies of H5N1 influenza virus resistance to oseltamivir. Protein Sci 18(4):707–715
Shu M, Lin Z, Zhang Y, Wu Y, Mei H, Jiang Y (2011) Molecular dynamics simulation of oseltamivir resistance in neuraminidase of avian influenza H5N1 virus. J Mol Model 17(3):587–592
Han N, Liu X, Mu Y 2012 Exploring the mechanism of zanamivir resistance in a neuraminidase mutant: Mol Dyn Stud. 2012
Woods CJ, Malaisree M, Long B, McIntosh-Smith S, Mulholland AJ (2013) Computational assay of H7N9 influenza neuraminidase reveals R292K mutation reduces drug binding affinity. Sci Rep 3(1):1–6
Thai K-M, Le D-P, Tran T-D, Le M-T (2015) Computational assay of Zanamivir binding affinity with original and mutant influenza neuraminidase 9 using molecular docking. J Theor Biol 385:31–39
Karthick V, Ramanathan K (2013) Virtual screening for oseltamivir-resistant a (H5N1) influenza neuraminidase from traditional Chinese medicine database: a combined molecular docking with molecular dynamics approach. Springerplus 2(1):1–10
Ren J, Huang J, Yang B, Lin S, Li J, Liao H et al (2019) Docking and molecular dynamics: simulation of the inhibition of H5N1 influenza virus (Anhui 2005) neuraminidase (NA) by chlorogenic acid (CHA). Int J Clin Exp Med 12(8):9815–9823
Nguyen H, Tran T, Fukunishi Y, Higo J, Nakamura H, Le L (2015) Computational study of drug binding affinity to influenza A Neuraminidase using smooth reaction path generation (SRPG) method. J Chem Inf Model 55(9):1936–1943
Do P-C, Lee EH, Le L (2018) Steered molecular dynamics simulation in rational drug design. J Chem Inf Model 58(8):1473–1482
Mai BK, Viet MH, Li MS (2010) Top leads for swine influenza A/H1N1 virus revealed by steered molecular dynamics approach. J Chem Inf Model 50(12):2236–2247
Mai BK, Li MS (2011) Neuraminidase inhibitor R-125489–a promising drug for treating influenza virus: steered molecular dynamics approach. Biochem Biophys Res Commun 410(3):688–691
Nguyen H, Nguyen HL, Linh HQ, Nguyen MT (2018) Binding affinity of the L-742,001 inhibitor to the endonuclease domain of A/H1N1/PA influenza virus variants: molecular simulation approaches. Chem Phys 500:26–36
Ballabh B, Chaurasia OP (2007) Traditional medicinal plants of cold desert Ladakh—used in treatment of cold, cough and fever. J Ethnopharmacol 112(2):341–349
Lee S-J, Umano K, Shibamoto T, Lee K-G 2005 Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91(1):131–7.
Narayanan MM, Nair CB, Sanjeeva SK, Rao PVS, Pullela PK, Barrow CJ (2013) Design of multiligand inhibitors for the swine flu H1N1 neuraminidase binding site. Adv Appl Bioinforma Chem AABC 6:47
Rains C, Bryson HM (1995) Topical Capsaicin. Drugs Aging 7(4):317–328
Gevorgyan A, Segboer C, Gorissen R, van Drunen CM, Fokkens W 2015 Capsaicin for non‐allergic rhinitis. Cochrane Database Syst Rev. 2015;(7).
Ternesten-Hasséus E, Johansson E-L, Millqvist E (2015) Cough reduction using capsaicin. Respir Med 109(1):27–37
Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM et al (2006) The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443(7107):45–49
Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA et al (2008) Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 453(7199):1258–1261
DeLano WL. Pymol: an open-source molecular graphics tool 2002 CCP4 Newsl protein Crystallogr. 2002;40(1):82–92.
DeLano WL. PyMOL. 2002.
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4(1):1–17
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Páll S, Abraham MJ, Kutzner C, Hess B, Lindahl E 2014 Tackling exascale software challenges in molecular dynamics simulations with GROMACS. In: International conference on exascale applications and software. Springer; 2014. p. 3–27.
Brooks BR, Brooks CL III, Mackerell AD Jr, Nilsson L, Petrella RJ, Roux B et al (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30(10):1545–1614
Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J 1981 Interaction models for water in relation to protein hydration. In: Intermolecular forces. Springer; 1981. p. 331–42.
Hockney RW, Goel SP, Eastwood JW (1974) Quiet high-resolution computer models of a plasma. J Comput Phys 14(2):148–158
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18(12):1463–1472
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J Chem Phys 98(12):10089–10092
He S, Maibaum L (2018) Identifying the onset of phase separation in quaternary lipid bilayer systems from coarse-grained simulations. J Phys Chem B 122(14):3961–3973
Berendsen HJC, van Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690
Petřek M, Otyepka M, Banáš P, Košinová P, Koča J, Damborský J (2006) CAVER: a new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinformatics 7(1):1–9
Srinivasan J, Cheatham TE, Cieplak P, Kollman PA, Case DA (1998) Continuum solvent studies of the stability of DNA, RNA, and phosphoramidate− DNA helices. J Am Chem Soc 120(37):9401–9409
Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L et al (2000) Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res 33(12):889–897
Author information
Authors and Affiliations
Contributions
N/A.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sedighpour, D., Taghizadeh, H. The effects of mutation on the drug binding affinity of Neuraminidase: case study of Capsaicin using steered molecular dynamics simulation. J Mol Model 28, 36 (2022). https://doi.org/10.1007/s00894-021-05005-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-05005-7