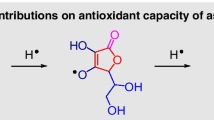
Abstract
In this study, we elucidated the formation of hydrogen bond between adrenaline (AD) and hydrogen sulfide utilizing computational studies. Six potential complexes were studied including geometrical parameters, energy, vibrational frequency, topological analysis, natural bond orbital (NBO), quantum theory of atoms in molecules (QTAIM), and NMR analysis. Moreover, these calculations were examined through DFT/ωB97XD/6-311G++(d,p) level. It was found that there are no indication on formation on hydrogen bonding between the two catecholic OHs where the one formed between the amino group and the hydroxyl oxygen atom of adrenaline monomer was broken in AS1 to form two new interactions namely SH...N and O1H1...S, while it retained in other complexes. Furthermore, the bond became stronger due to cooperativity in AS3 and AS6, for the presence of withdrawing effect of the phenyl ring, the H-bonds formed with the side chain oxygen atom. The adrenaline and H2S interaction was experimentally examined via FT-IR spectrometry and thin layer chromatography for confirmation of our theoretical study.

Graphical abstract












Similar content being viewed by others
References
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods2nd edn. Gaussian Inc., Pittsburgh
Hameed AJ, Ibrahim M, Elhaes H (2007) Computational notes on structural, electronic and QSAR properties of Fulleropyrrolidine-1-carbodithioic acid 2; 3 and 4-substituted-benzyl esters. J Mol Struct (THEOCHEM) 809:131–136
Ibrahim M, Osman O (2009) Spectroscopic analyses of cellulose: Fourier transform infrared and molecular modelling study. J Comput Theor Nanosci 6(5):1054–1058
Youness RA, Taha MA, Ibrahim MA (2017) Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J Mol Struct 1150:188–195
Essa AH, Ibrahim M, Hameed AJ, Al-Masoudi NA (2008) Theoretical investigation of 3′-subtituted-2′-3′-dideoxythymidines related to AZT. QSAR infrared and substituent electronic effect studies. ARKIVOC xiii:255–265
Ibrahim M, Osman O, Mahmoud A-A (2011) Spectroscopic analyses of cellulose and chitosan: FTIR and modeling approach. J Comput Theor Nanosci 8:117–123
Fahim AM, Shalaby M, Ibrahim M (2019) Microwave-assisted synthesis of novel 5-aminouracil-based compound with DFT calculations. J Mol Struct 1194:211–226
Mahmoud A-A, Osman O, Elhaes H, Ferretti M, Fakhry A, Ibrahim MA (2018) Computational analyses for the interaction between aspartic acid and iron. J Comput Theor Nanosci 15:470–473
Youness RA, Taha MA, Elhaes H, Ibrahim M (2017) Molecular modeling, FTIR spectral characterization and mechanical properties of carbonated-hydroxyapatite prepared by mechanochemical synthesis. Mater Chem Phys 190:209–218
Ibrahim HS, Ammar NS, Soylak M, Ibrahim M (2012) Removal of Cd(II) and Pb(II) from aqueous solution using dried water hyacinth as a biosorbent. Spectrochim Acta A 96:413–420
Ammar NS, Elhaes H, Ibrahim HS, El-hotaby W, Ibrahim MA (2014) A novel structure for removal of pollutants from wastewater. Spectrochim Acta A 121C:216–223
Dacrory S, Fahim AM (2020) Synthesis, anti-proliferative activity, computational studies of tetrazole cellulose utilizing different homogenous catalyst. Carbohydr Polym 229:115537. https://doi.org/10.1016/j.carbpol.2019.115537
Cannon WB (1915) Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. D Appleton & Company, New York
El-Melih AM, Al Shoaibi A, Gupta AK (2017) Reformation of hydrogen sulfide to hydrogen in the presence of xylene. Appl Energy 203:403–411
Butz P, Kroemer RT, Macleod NA, Simons JP (2001) Conformational preferences of neurotransmitters: ephedrine and its diastereoisomer, pseudoephedrine. J Phys Chem A 105:544–551
Alonso JL, Sanz ME, Lopez JC, Cortijo V (2009) Conformational behavior of norephedrine, ephedrine, and pseudoephedrine. J Am Chem Soc 131:4320–4326
Butz P, Kroemer RT, Macleod NA, Simons JP (2002) Hydration of neurotransmitters: a spectroscopic and computational study of ephedrine and its diastereoisomer pseudoephedrine. Phys Chem Chem Phys 4:3566–3574
Snoek LC, van Mourik T, Carcabal P, Simons JP (2003) Neurotransmitters in the gas phase: hydrated noradrenaline. Phys Chem Chem Phys 5:4519–4526
van Mourik T (2004) The shape of neurotransmitters in the gas phase: a theoretical study of adrenaline, pseudoadrenaline, and hydrated adrenaline. Phys Chem Chem Phys 6:2827–2837
Macleod NA, Simons JP (2006) Infrared photodissociation spectroscopy of protonated neurotransmitters in the gas phase. Mol Phys 104:3317–3328
Vaden TD, de Boer T, MacLeod NA, Marzluff EM, Simons JP, Snoek LC (2007) Infrared spectroscopy and structure of photochemically protonated biomolecules in the gas phase: a noradrenaline analogue, lysine and alanyl alanine. Phys Chem Chem Phys 9:2549–2555
Alagona G, Ghio C (2007) Competitive H-bonds in vacuo and in aqueous solution for N-protonated adrenaline and its monohydrated complexes. J Mol Struct (THEOCHEM) 811:223–240
Wang Y, Wang Z, Pan J, Liu Y (2019) Removal of gaseous hydrogen sulfide using Fenton reagent in a spraying reactor. Fuel 239:70–75
Hancock JT (2019) Hydrogen sulfide splitting using solar energy and hematite photo-anodes. Environ Exp Bot 161:50–56
Carcabal P, Snoek LC, Van Mourik T (2005) A computational and spectroscopic study of the gas-phase conformers of adrenaline. Mol Phys 103:1633–1639
Kolluru GK, Shen X, Bir SC, Kevil CG (2013) Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 35(30):5–20
Zhang Z, Jin S, XuTenga XD, Chen Y, Wu Y (2017) Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 67:10–25
Filogonio R, Crossley DA (2019) Long term effects of chronic prenatal exposure to hypercarbia on organ growth and cardiovascular responses to adrenaline and hypoxia in common snapping turtles. Comp Biochem Physiol A Mol Integ Physiol 234:10–17
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc., Wallingford
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-8. J Chem Phys 72:5639–5648
Wang H, Huang Z, Shen T, Guo L (2012) Hydrogen-bonding interactions in adrenaline–water complexes: DFT and QTAIM studies of structures, properties, and topologies. J Mol Model 18:3113–3123
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comp Chem 33:580–592
HumphreyW DA, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Arunan E, Desiraju GR, Klein RA, Sadlej J, Shiener S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ (1637-1641). Pure Appl Chem 83(8):2011
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, New York
Popelier PLA (2000) Atoms in molecules: an introduction. Pearson Education, Harlow
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Carroll MT, Bader RFW (1988) An analysis of the hydrogen bond in BASE–HF complexes using the theory of atoms in molecules. Mol Phys 65:695–722
Carroll MT, Chang C, Bader RFW (1988) Prediction of the structures of hydrogen bonded complexes using the Laplacian of the charge density. Mol Phys 63:387–405
Bader RFW, Chang C (1989) Properties of atoms in molecules: electrophilic aromatic substitution. J Phys Chem 93:2946–2956
Popelier PLA, Bader RFW (1992) The existence of an intramolecular C–H…O hydrogen bond in creatine and carbamoyl sarcosine. Chem Phys Lett 189:542–548
Koch U (1995) Popelier PLA characterization of C–H–O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Reed AE, Weinhold F, Curtiss LA, Pochatko DJ (1986) Natural bond orbital analysis of molecular interactions: theoretical studies of binary complexes of HF, H2O, NH3, N2, O2, F2, CO, and CO2 with HF, H2O, and NH3. J Chem Phys 84:5687–5705
Scheiner S (2016) Interpretation of spectroscopic markers of hydrogen bonds. Chemphyschem 17(14):2263–2271
Scheiner S (2016) Assessment of the presence and strength of H-bonds by means of corrected NMR. Molecules 21:1426
Johnson ER, Keinan S, Mori-Sanchez P et al (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, A., Fahim, A.M. & Ibrahim, M.A. Theoretical investigation on hydrogen bond interaction between adrenaline and hydrogen sulfide. J Mol Model 26, 354 (2020). https://doi.org/10.1007/s00894-020-04602-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04602-2