Abstract
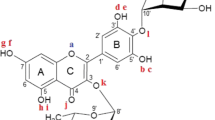
In this work, we present a computational investigation on the structure and energetics of eleocarpanthraquinone, a newly isolated polyphenolic anthrone-antraquinone. Properties such as bond lengths, angles, atomic charges, bond dissociation enthalpies (BDEs), and ionization potential (IP) were determined through the use of density functional theory (DFT). The B3LYP and M06-2X exchange-correlation functionals were employed along with the 6-31+G(d,p), 6-31+ +G(d,p), and 6-311+G(d,p) basis sets for performing computations in the gas-phase, water, methanol, and ethanol. The conformation presenting all the hydroxyl groups undergoing hydrogen-bond interactions with neighboring oxygen atoms (conformation 5) was assigned as the most stable structure while its counterpart presenting no hydrogen-bond interaction was found to be 36.45 kcal/mol less stable than conformation 5 in the potential energy surface probed at the B3LYP/6-311+G(d,p) level of theory in the gas-phase, for instance. More importantly, the lowest O–H bond dissociation enthalpy was determined to be 93.80 kcal/mol at the B3LYP/6-311+G(d,p) level of theory in water against the 146.58 kcal/mol regarding the IP computed at the same approach, suggesting the hydrogen atom transfer mechanism as being preferred over the single electron transfer mechanism in regards to the antioxidant potential for the case of eleocarpanthraquinone; the same conclusion was drawn from the outcomes of all the other approaches used.




Similar content being viewed by others
References
All natural (2007) . Nat Chem Biol 3:351. https://doi.org/10.1038/nchembio0707-351
Hanson JR (2003) Natural products: the secondary metabolite. Royal Society of Chemistry, Cambridge
Toric J, Markovic AK, Brala CJ, Barbaric M (2019) Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm 69:461–482
Sun LR, Zhou W, Zhang HM, Guo QS, Yang W, Li BJ, Sun ZH, Gao SH, Cui RJ (2019) Modulation of multiple signaling pathways of the plant-derived natural products in cancer. Front Oncol 9:1153
Huang XM, Yang ZJ, Xie Q, Zhang ZK, Zhang H, Ma JY (2019) Natural products for treating colorectal cancer: a mechanistic review. Biomed Pharmacother 117:109142
Joshi SS, Howell AB, D’Souza DH (2019) Antiviral effects of blueberry proanthocyanidins against Aichi virus. Food Microbiol 82:202–208
Martillanes S, Rocha-Pimienta J, Gil MV, Ayuso-Yuste MC, Delgado-Adamez J (2019) Antioxidant and antimicrobial evaluation of rice bran (Oryza sativa L.) extracts in a mayonnaise-type emulsion. Food Chem 308:125633
Plaza M, Kariuki J, Turner C (2014) Quantification of individual phenolic compounds: contribution to antioxidant capacity in apple: a novel analytical tool based on liquid chromatography with diode array, electrochemical, and charged aerosol detection. J Agric Food Chem 62:409–418
Bertha CT, Alberto SBJ, Tovar J, Sáyago-Ayerdi S G, Zamora-Gasga VM (2019) In vitro gastrointestinal digestion of mango by-product snacks: potential absorption of polyphenols and antioxidant capacity. Int J Food Sci Tech 54:3091–3098
Cherubim DJL, Martins CVB, Fariña L O, de Lucca RAS (2019) Polyphenols as natural antioxidants in cosmetics applications. J Cosmet Dermatol 19:33–37
Dzah CS, Duan Y, Zhang H, Boateng NAS, Ma H (2020) Latest developments in polyphenol recovery and purification from plant by-products: a review. Trends Food Sci Technol 99:375–388
Afsar T, Razak S, Shabbir M, Khan MR (2018) Antioxidant activity of polyphenolic compounds isolated from ethyl-acetate fraction of Acacia hydaspica R. Parker Chem Cent J 12:5
Olate-Gallegos C, Barriga A, Vergara C, Fredes C, García P, Giméenez B, Robert P (2019) Identification of polyphenols from Chilean Brown seaweeds extracts by LC-DAD-ESI-MS/MS. J Aquat Food Prod Technol 28:375–391
Biniari K, Xenaki M, Daskalakis I, Rusjan D, Bouza D, Stavrakaki M (2020) Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem 307:125518
Mannino G, Perrone A, Campobenedetto C, Schittone A, Bertea CM, Gentile C (2020) Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: a new source of nutraceuticals. Food Chem 307:125515
Lachowicz S, Seliga L, Pluta S (2020) Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem 305:125430
Nenadis N, Sigalas MP (2008) A DFT study on the radical scavenging activity of maritimetin and related aurones. J Phys Chem A 112:12196–12202
Mendes RA, e Silva BLS, Takeara R, Freitas RG, Brown A, de Souza GLC (2018) Probing the antioxidant potential of phloretin and phlorizin through a computational investigation. J Mol Model 24:101
Mendes RA, Almeida SKC, Soares IN, Barboza CA, Freitas RG, Brown A, de Souza GLC (2018) A computational investigation on the antioxidant potential of myricetin 3,4\(^{\prime }\)-di-O-α-L-rhamnopyranoside. J Mol Model 24:133
Maciel EN, Almeida SKC, da Silva SC, de Souza GLC (2018) Examining the reaction between antioxidant compounds and 2,2-diphenyl-1-picrylhydrazyl (DPPH) through a computational investigation. J Mol Model 24:218
Maciel EN, Soares IN, da Silva SC, de Souza GLC (2019) A computational study on the reaction between fisetin and 2,2-diphenyl-1-picrylhydrazyl (DPPH). J Mol Model 25:103
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem A 108:92–96
Deepha V, Praveena R, Sivakumar R, Sadasivam K (2014) Experimental and theoretical investigations on the antioxidant activity of isoorientin from Crotalaria globosa. Spectrochim Acta A 121:737–745
Stepanić V, Troselj KG, Lucić B, Marković Z, Amić D (2013) Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem 141:1562–1570
Gomes JRB, da Silva MAVR (2003) Gas-phase thermodynamic properties of dichlorophenols determined from density functional theory calculations. J Phys Chem A 107:869–874
Amić D, Stepanić W, Lučić R, Marković Z, Dmitrić Marković J M (2013) PM6 study of free radical scavenging mechanisms of flavonoids: why does OH bond dissociation enthalpy effectively represent free radical scavenging activity. J Mol Model 19:2593–2603
Giacomelli C, Miranda FS, Goncalves NS, Spinelli A (2004) Antioxidant activity of phenolic and related compounds: a density functional theory study on the O-H bond dissociation enthalpy. Redox Rep 9:263
Chen Y, Xiao H, Zheng J, Liang G (2015) Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: an experimental and theoretical evaluation. PLoS One 10:e0121276
Zheng YZ, Chen DF, Deng G, Guo R, Fu ZM (2018) The surrounding environments on the structure and antioxidative activity of luteolin. J Mol Model 24:149
Musialik M, Kuzmicz R, Pawlowski TS, Litwinienko G (2009) Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J Org Chem 74:2699–2709
Musialik M, Litwinienko G (2005) Scavenging of dpph radicals by vitamin E is accelerated by its partial ionization: the role of sequential proton loss electron transfer. Org Lett 7:4951–4954
Kumar KS, Kumaresan R (2013) . Mol Simulat 39:72
Mendes RA, Almeida SKC, Soares IN, Barboza CA, Freitas RG, Brown A, de Souza GLC (2019) A computational investigation on the antioxidant potential of myricetin 3\(^{\prime }\)-O-α-L-rhamnopyranoside and 4\(^{\prime }\)-O-α-L-rhamnopyranoside. J Mol Model 25:89
Varenyi S, Chrenova S, Lukac M (2019) Analysis of antioxidant activity of polyphenolic compounds of lichens and liverworts using thin layer chromatography with detection by 2,2-diphenyl-1-picrylhydrazyl. Chem Listy 113:53–57
Melo JG, Rodrigues MD, Nascimento SC, Amorim ELC, Albuquerque UP (2017) Cytotoxicity of plants from the Brazilian semi-arid region: a comparison of different selection approaches. S Afr J Bot 113:47–53
Lorenzi H, Bacher L, Lacerda M, Sartori S (2006) Brazilian fruits and cultivated exotics. Instituto Plantarum de Estudos da Flora LTDA. ISBN-10: 8586714240
Nishijima C, Sommerfeld O, Honda N, Brum R, Coelho R, Hiruma-Lima C (2009) Antinociceptive activity of methanolic extract from Rhamnidium elaeocarpum barks. Planta Med 75:934
de Sousa PT Jr (2019) (private communication)
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Zhao Y, Truhlar DG (2006) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Account 120:215–241
Rassolov V, Pople JA, Ratner M, Redfern PC, Curtiss LA (2001) 6-31G* basis set for third-row atoms. J Comput Chem 22:976–984
Binkley JS, Pople JA, Hehre WJ (1980) Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J Am Chem Soc 102:939–946
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261
La Rocca MV, Rutkowski M, Ringeissen S, Gomar J, Frantz MC, Ngom S, Adamo C (2016) Benchmarking the DFT methodology for assessing antioxidant-related properties: quercetin and edaravone as case studies. J Mol Model 22:250–260
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum models of solvation. I. General formalism. J Chem Phys 132:114110
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford, Gaussian 09, Revision D.02
de Souza GLC, de Oliveira LMF, Vicari RG, Brown A (2016) A DFT investigation on the structural and antioxidant properties of new isolated interglycosidic O-(1-3) linkage flavonols. J Mol Model 22:100–109
Yan M, Gong J, Shen P, Yang C (2014) The theory investigation for the antioxidant activity of phloretin: a comparation with naringenin. AMM 513:359–362
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922
Acknowledgments
The authors thank Professor Kirk A. Peterson (Washington State University) for providing part of the computational resources used in this work.
Funding
Gabriel L. C. de Souza received funding from the Brazilian agency CNPq (grants 204748/2018-6 and 306433/2019-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection XX - Brazilian Symposium of Theoretical Chemistry (SBQT2019)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Santos, J.L.F., Kauffmann, A.C., da Silva, S.C. et al. Probing structural properties and antioxidant activity mechanisms for eleocarpanthraquinone. J Mol Model 26, 233 (2020). https://doi.org/10.1007/s00894-020-04469-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04469-3