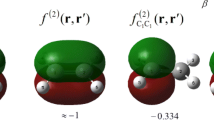
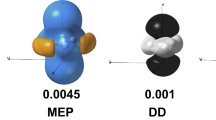
Abstract
An alternative way of calculating the Fukui function and the partial derivative of second order of the electronic density with respect to the number of electrons N is presented, the new formulas agree with the usual ones but only in cases without degeneracy. The new operative formulas are more general than the previous ones and are the right ones for those problematic cases where one or both of the frontier molecular orbitals are degenerate. Finally, we present a new way of applying the finite difference approximation that leads to more realistic results than the usual formulas.

A new way of calculating the Fukui function is presented that results in a new operative formula of the function. It has also been obtained the partial derivative of second order of the electronic density with respect to the number of electrons N, and it agree with the usual formula of the dual descriptor function but only in cases without degeneration.




Similar content being viewed by others
References
Yang WT, Parr RG, Pucci R (1984) Electron density, Kohn-sham frontier orbitals, and Fukui functions. J Chem Phys 81:2862–2863
Par RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106:4049
Ayers PW, Levy M (2000) Density functional approach to the frontierelectron theory of chemical reactivity. Theor Chem Accounts 103:353
Ayers PW, Yang WT, Bartolotti LJ (2009) The Fukui function. In: Chattaraj PK (ed) Chemical reactivity theory: a density functional view. CRC, Boca Raton, p 255
Yang W, Parr RG (1985) Hardness, softness, and the Fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci USA 82:6723
Chandra AK, Nguyen MT (2008) Fukui function and local softness. In: Chattaraj PK (ed) Chemical reactivity theory: a density-functional view. Taylor and Francis, New York, pp 163–178
Geerlings P, Proft FD, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874
Parr R, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, Oxford
Perdew JP, Parr RG, Levy M, Balduz JL (1982) Density-functional theory for fractional particle number: derivative discontinuities of the energy. Phys Rev Lett 49:1691–1694
Yang WT, Zhang YK, Ayers PW (2000) Degenerate ground states and fractional number of electrons in density and reduced density matrix functional theory. Phys Rev Lett 84:5172–5175
Ayers PW (2008) The continuity of the energy and other molecular properties with respect to the number of electrons. J Math Chem 43:285–303
Gázquez JL (2009) Chemical reactivity concepts in density functional theory. In: Chattaraj PK (ed) Chemical reactivity theory: A density functional view. CRC, Boca Raton, p 7
Morell C, Gázquez JL, Vela A, Guegan F, Chermette H (2014) Revisiting electroaccepting and electrodonating powers: proposals for local electrophilicity and local nucleophilicity descriptors. Phys Chem Chem Phys 16:26832
Robles A, Franco-Pérez M, Gázquez JL, Cárdenas C, Fuentealba P (2018) Local electrophilicity. J Mol Model 24:245
Morell C, Grand A, Toro-Labbe A (2005) New dual descriptor for chemical reactivity. J Phys Chem A 109:205–212
Morell C, Grand A, Toro-Labbe A (2006) Theoretical support for using the Δf(r) descriptor. Chem Phys Lett 425:342–346
De Proft F, Ayers PW, Fias S, Geerlings P (2006) Woodward-Hoffmann rules in density functional theory: initial hardness response. J Chem Phys 125:214101–214109
Ayers PW, Morell C, De Proft F, Geerlings P (2007) Understanding the Woodward–Hoffmann rules by using changes in Electron density. Chem Eur J 13:8240–8247
Cárdenas AC, Ayers PW, Cedillo A (2011) Reactivity indicators for degenerate states in the density-functional theoretic chemical reactivity theory. J Chem Phys 134:174103–13
Bultinck P, Cardenas C, Fuentealba P, Johnson PA, Ayers PW (2013) Atomic charges and the electrostatic potential are ill-defined in degenerate ground states. J Chem Theory Comp 9:4779–4788
Bultinck P, Cardenas C, Fuentealba P, Johnson PA, Ayers PW (2014) How to compute the Fukui matrix and function for systems with (quasi-)degenerate states. J Chem Theory Comp 10:202–210
Bultinck P, Jayatilaka D, Cardenas C (2015) A problematic issue for atoms in molecules: impact of (quasi-)degenerate states on quantum theory atoms in molecules and Hirshfeld-I properties. Comput Theor Chem 1053:106–111
Martínez-Araya JI (2016) A generalized operational formula based on Total electronic densities to obtain 3D pictures of the dual descriptor to reveal nucleophilic and electrophilic sites accurately on closed-Shell molecules. J Comput Chem 37:2279–2303
Martínez-Araya JI (2015) Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions? J Math Chem 53:451–465
Becke AD (1993) Density-functional thermochemistry. III the role of exact exchange. J Chem Phys 98:5648–5652
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods. 25. Supplementary functions for gaussian basis sets. J Chem Phys 80:3265–3269
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford CT
Sánchez-Márquez J, Zorrilla D, Sánchez-Coronilla AM, de los Santos D, Navas J, Fernández-Lorenzo C, Alcántara R, Martín-Calleja J (2014) Introducing UCA-FUKUI software: reactivity-index calculations. J Mol Model 20:2492
Bultinck P et al (2007) Critical thoughts on computing atom condensed Fukui functions. J Chem Phys 127:034102–034111
Nalewajski RF, Parr RG (2000) Information theory, atoms in molecules, and molecular similarity. Proc Natl Acad Sci USA 97:8879–8882
Heidar-Zadeh F, Ayers PW, Verstraelen T, Vinogradov I, Vöhringer-Martinez E, Bultinck P (2018) Information-theoretic approaches to atoms-in-molecules: Hirshfeld family of partitioning schemes. J Phys Chem A 122:4219–4245
Roy RK, Pal S, Hirao K (1999) On non-negativity of Fukui function indices. J Chem Phys 110:8236–8245
Yang WT, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chem Accounts 44:129–138
Ritchie JP (1985) Electron density distribution analysis for nitromethane, nitromethide, and nitramide. J Am Chem Soc 107:1829–1837
Ritchie JP, Bachrach SM (1987) Some methods and applications of electron density distribution analysis. J Comp Chem 8:499–509
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions I. J Chem Phys 23:1833
Reed AE, Weinhold F (1983) Natural bond orbital analysis of near-Hartree-Fock water dimer. J Chem Phys 78:4066
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735
Reed AE, Weinhold F (1985) Natural localized molecular orbitals. J Chem Phys 83:1736
Orozco-Valencia U, Gazquez JL, Vela A (2018) Global and local charge transfer in electron donor-acceptor complexes. J Mol Model 24:250
Orozco-Valencia U, Gazquez JL, Vela A (2018) Role of reaction conditions in the global and local two parabolas charge transfer model. J Phys Chem A 122:1796–1806
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Parr RG, Bartolotti LJ (1982) On the geometric mean principle for electronegativity equalization. J Am Chem Soc 104:3801–3803
Heidar-Zadeh F (2016) When is the Fukui function not normalized? The danger of inconsistent energy interpolation models in density functional theory. J Chem Theory Comp. 12:5777–5787
Heidar-Zadeh F, Richer M, Fias S, Miranda-Quintana RA, Chan M, Franco-Pérez M, González-Espinoza CE, Kim TD, Lanssens C, Patel AHG, Yang XD, Vöhringer-Martinez E, Cárdenas C, Verstraelen T, Ayers PW (2016) Chem Phys Lett 660:307–312
Franco-Perez M, Gá́zquez JL, Ayers PW, Vela A (2018) Thermodynamic justification for the parabolic model for reactivity indicators with respect to Electron number and a rigorous definition for the Electrophilicity: the essential role played by the electronic entropy. J Chem Theory Comp 14:597–606
Dennington R, Keith T, Millam J (2009) Gauss View 5.0. Semichem Inc, Shawnee Mission, KS 7
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. WILEY-VCH, Weinham
Bader RFW (2005) The quantum mechanical basis for conceptual chemistry. Monatsh Chem 136:819–854
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys 112:6532
Sánchez-Márquez J (2016) Introducing new reactivity descriptors: “bond reactivity indices.” comparison of the new definitions and atomic reactivity indices. J Chem Phys 145:194105–194112
Sánchez-Márquez J, Zorrilla D, García V, Fernández M (2018) Introducing a new bond reactivity index: Philicities for natural bond orbitals. J Mol Model 24:25
Sánchez-Márquez J, Zorrilla D, García V, Fernández M (2018) Introducing a new methodology for the calculation of local philicity and multiphilic descriptor: an alternative to the finite difference approximation. Mol Phys 116:1737–1748
Acknowledgment
Calculations were performed through CICA (Centro Informático Científico de Andalucía).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 749 kb)
Rights and permissions
About this article
Cite this article
Sánchez-Márquez, J. New advances in conceptual-DFT: an alternative way to calculate the Fukui function and dual descriptor. J Mol Model 25, 123 (2019). https://doi.org/10.1007/s00894-019-4000-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4000-0