Abstract
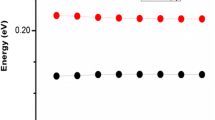
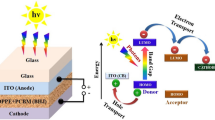
Some important optoelectronic properties of naphtho[2,1-b:6,5-b′]difuran (DPNDF) and its two derivatives have been limelighted by applying the density functional theory (DFT). Due to their low cost, high stability and earth abundance, the DPNDF and its derivatives are considered as potential organic semiconductor materials for their optoelectronics applications. Highly proficient derivatives are obtained systematically by attaching –CN (cyanide) to DPNDF at various sites. Our calculations indicate that DPNDF has a wide and direct band gap with an energy gap of 3.157 eV. Whereas the band gaps of its derivatives are found to be decreased by 88 meV for derivative “a” and 300 meV for derivative “b” as a consequence of p orbitals present in C and N atoms in derivative structures. The narrowing of the energy gap and density of states for the derivatives of DPNDF in the present investigation suggest that energy gap can be engineered for desirable optoelectronic applications via derivatives designing. Furthermore, their obtained results for optical parameters such as the dielectric and conductivity functions, reflectivity, refractive index, and the extinction coefficients endorses their aptness for optoelectronic applications.

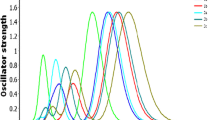
Real part of dielectric function for derivative “b”








Similar content being viewed by others
References
Ren G, Wu P-T, Jenekhe SA (2010) Enhanced performance of bulk heterojunction solar cells using block copoly (3-alkylthiophene) s. Chem Mater 22(6):2020–2026
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109(11):5868–5923
Dennler G, Scharber MC, Brabec CJ (2009) Polymer-fullerene bulk-heterojunction solar cells. Adv Mater 21(13):1323–1338
Grimsdale AC, Leok Chan K, Martin RE, Jokisz PG, Holmes AB (2009) Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem Rev 109(3):897–1091
Shirota YKageyama H (2007) Charge carrier transporting molecular materials and their applications in devices. Chem Rev 107(4):953–1010
Berggren M, Inganäs O, Gustafsson G, Rasmusson J, Andersson MR, Hjertberg T, Wennerström O (1994) Light-emitting diodes with variable colours from polymer blends. Nature 372:444–446
Wen YLiu Y (2010) Recent progress in n-channel organic thin-film transistors. Adv Mater 22(12):1331–1345
DiBenedetto SA, Facchetti A, Ratner MA, Marks TJ (2009) Molecular self-assembled monolayers and multilayers for organic and unconventional inorganic thin-film transistor applications. Adv Mater 21(14/15):1407–1433
Zaumseil JSirringhaus H (2007) Electron and ambipolar transport in organic field-effect transistors. Chem Rev 107(4):1296–1323
Muccini M (2006) A bright future for organic field-effect transistors. Nat Mater 5(8):605–613
Boto AAlvarez L (2011) Furan and its derivatives. Heterocycles in natural product synthesis: 97–152.
Majumdar KCChattopadhyay SK (2011) Heterocycles in natural product synthesis. Wiley
Brown RC (2005) Developments in furan syntheses. Angew Chem Int Ed 44(6):850–852
Bunz UH (2010) α‐Oligofurans: molecules without a twist. Angew Chem Int Ed 49(30):5037–5040
Niimi K, Mori H, Miyazaki E, Osaka I, Kakizoe H, Takimiya K, Adachi C (2012) [2,2[prime or minute]]Bi[naphtho[2,3-b]furanyl]: a versatile organic semiconductor with a furan-furan junction. Chem Commun 48(47):5892–5894
Huo L, Huang Y, Fan B, Guo X, Jing Y, Zhang M, Li Y, Hou J (2012) Synthesis of a 4, 8-dialkoxy-benzo [1, 2-b: 4, 5-b′] difuran unit and its application in photovoltaic polymer. Chem Commun 48(27):3318–3320
Gidron O, Diskin-Posner Y, Bendikov M (2010) α-Oligofurans. J Am Chem Soc 132(7):2148–2150
Tsuji H, Mitsui C, Ilies L, Sato Y, Nakamura E (2007) Synthesis and properties of 2, 3, 6, 7-tetraarylbenzo [1, 2-b: 4, 5-b′] difurans as hole-transporting material. J Am Chem Soc 129(39):11902–11903
Distefano G, Jones D, Guerra M, Favaretto L, Modelli A, Mengoli G (1991) Determination of the electronic structure of oligofurans and extrapolation to polyfuran. J Phys Chem 95(24):9746–9753
Mitsui C, Soeda J, Miwa K, Tsuji H, Takeya J, Nakamura E (2012) Naphtho[2,1-b:6,5-b′]difuran: a versatile motif available for solution-processed single-crystal organic field-effect transistors with high hole mobility. J Am Chem Soc 134(12):5448–5451
Dang D, Zhou P, Zhong J, Fan J, Wang Z, Wang Y et al (2014) Novel wide band-gap polymer utilizing fused hetero-aromatic unit for efficient polymer solar cells and field-effect transistors. Polymer 55(26):6708–6716
Kröger M, Hamwi S, Meyer J, Riedl T, Kowalsky W, Kahn A (2009) P-type doping of organic wide band gap materials by transition metal oxides: a case-study on molybdenum trioxide. Org Electron 10(5):932–938
Li W, Furlan A, Roelofs WSC, Hendriks KH, van Pruissen GWP, Wienk MM, Janssen RAJ (2014) Wide band gap diketopyrrolopyrrole-based conjugated polymers incorporating biphenyl units applied in polymer solar cells. Chem Commun 50(6):679–681
Wang Q, Sun B, Aziz H (2014) Exciton–polaron-induced aggregation of wide-bandgap materials and its implication on the electroluminescence stability of phosphorescent organic light-emitting devices. Adv Funct Mater 24(20):2975–2985
Someya T, Sekitani T, Iba S, Kato Y, Kawaguchi H, Sakurai T (2004) A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc Natl Acad Sci U S A 101(27):9966–9970
Chaudhry AR, Ahmed R, Irfan A, Shaari A, Maarof H, Al-Sehemi AG (2014) First principles investigations of electronic, photoluminescence and charge transfer properties of the naphtho[2,1-b:6,5-b′]difuran and its derivatives for OFET. Sains Malays 43(6):867–875
Chaudhry AR, Ahmed R, Irfan A, Shaari A, Al-Sehemi AG (2014) Effects of electron withdrawing groups on transfer integrals, mobility, electronic and photo-physical properties of naphtho[2,1-b:6,5-b′]difuran derivatives: a theoretical study. Sci Adv Mater 6(8):1727–1739
Mohamad M, Ahmed R, Shaari A, Goumri-Said S (2015) First principles investigations of vinazene molecule and molecular crystal: a prospective candidate for organic photovoltaic applications. J Mol Model 21(2):1–7
Dennington R, Keith T, Millam J (2009) GaussView, version 5. Semichem Inc, Shawnee Mission, KS
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al. (2009) Gaussian 09 revision A.02. Gaussian Inc, Wallingford
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98(45):11623–11627
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789
Cho E, Risko C, Kim D, Gysel R, Cates Miller N, Breiby DW et al (2012) Three-dimensional packing structure and electronic properties of biaxially oriented poly(2,5-bis(3-alkylthiophene-2-yl)thieno[3,2-b]thiophene) films. J Am Chem Soc 134(14):6177–6190
Sajoto T, Tiwari SP, Li H, Risko C, Barlow S, Zhang Q et al (2012) Synthesis and characterization of naphthalene diimide/diethynylbenzene copolymers. Polymer 53(5):1072–1078
Miyata Y, Nishinaga T, Komatsu K (2005) Synthesis and structural, electronic, and optical properties of oligo(thienylfuran)s in comparison with oligothiophenes and oligofurans. J Org Chem 70(4):1147–1153
Miyata Y, Terayama M, Minari T, Nishinaga T, Nemoto T, Isoda S, Komatsu K (2007) Synthesis of oligo(thienylfuran)s with thiophene rings at both ends and their structural, electronic, and field-effect properties. Chem Asian J 2(12):1492–1504
Gidron O, Dadvand A, Sheynin Y, Bendikov M, Perepichka DF (2011) Towards “green” electronic materials. α-Oligofurans as semiconductors. Chem Commun 47(7):1976–1978
Wu C-C, Hung W-Y, Liu T-L, Zhang L-Z, Luh T-Y (2003) Hole-transport properties of a furan-containing oligoaryl. J Appl Phys 93(9):5465–5471
Irfan A, Al-Sehemi AG, Muhammad S, Zhang J (2011) Packing effect on the transfer integrals and mobility in α, α′-bis(dithieno[3,2-b:2′,3′-d]thiophene) (BDT) and its heteroatom-substituted analogues. Aust J Chem 64(12):1587–1592
Irfan A, Zhang J, Chang Y (2010) Theoretical investigations of the charge transfer properties of anthracene derivatives. Theor Chem Acc 127(5–6):587–594
Accelrys software Inc (2013) MaterialsStudio, version 7.0. Accelrys software Inc, San Diego
Baur WHKassner D (1992) The perils of Cc: comparing the frequencies of falsely assigned space groups with their general population. Acta Crystallogr Sect B: Struct Sci 48(4):356–369
Mayo SL, Olafson BD, Goddard WA (1990) DREIDING: a generic force field for molecular simulations. J Phys Chem 94(26):8897–8909
Liu J, Dong M, Qin Z, Wang J (2004) Grand canonical Monte Carlo simulation of benzene, cyclohexane and hexane sorption in AlPO4-5. J Mol Struct THEOCHEM 679(1–2):95–99
Klemm E, Wang J, Emig G (1998) A comparative study of the sorption of benzene and phenol in silicalite, HAlZSM-5 and NaAlZSM-5 by computer simulation. Microporous Mesoporous Mater 26(1–3):11–21
Fried JR, Weaver S (1998) Atomistic simulation of hydrocarbon diffusion in silicalite. Comput Mater Sci 11(4):277–293
Masunov A, Zorkii PM (1992) Donor-acceptor nature of specific nonbonded interactions of sulfur and halogen atoms. Influence on the geometry and packing of molecules. J Struct Chem 33(3):423–435
Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MI, Refson K, Payne MC (2005) First principles methods using CASTEP. Z Krist 220:567–570
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45(23):13244
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Erratum: Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 48(7):4978
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46(11):6671–6687
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41(11):7892
Laasonen K, Car R, Lee C, Vanderbilt D (1991) Implementation of ultrasoft pseudopotentials in ab initio molecular dynamics. Phys Rev B 43(8):6796
Yu ZG, Gong H, Wu P (2006) Lattice dynamics and electrical properties of wurtzite ZnO determined by a density functional theory method. J Cryst Growth 287(1):199–203
Hellmann H (1935) A new approximation method in the problem of many electrons. J Chem Phys 3(1):61
Laasonen K, Pasquarello A, Car R, Lee C, Vanderbilt D (1993) Car-Parrinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials. Phys Rev B 47(16):10142
Lee C, Vanderbilt D, Laasonen K, Car R, Parrinello M (1993) Ab initio studies on the structural and dynamical properties of ice. Phys Rev B 47(9):4863
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188
Press WH, Vetterling W, Teukolsky SA, Flannery BP, Greenwell Yanik E (1994) Numerical recipes in fortran—the art of scientific computing. SIAM Rev 36(1):149
Sun H, Ren P, Fried J (1998) The COMPASS force field: parameterization and validation for phosphazenes. Comput Theor Polym Sci 8(1):229–246
Chaudhry AR, Ahmed R, Irfan A, Shaari A, Al-Sehemi AG (2014) Effects of electron withdrawing groups on transfer integrals, mobility, electronic and photo-physical properties of the naphtho[2,1-b:6,5-b′]difuran derivatives: a theoretical study. Sci Adv Mater 6:1–13
Irfan A, Cui R, Zhang J, Nadeem M (2010) Designing of disubstituted derivatives of mer-Alq3: quantum theoretical study. Aust J Chem 63(8):1283–1289
Sato N, Seki K, Inokuchi H (1981) Polarization energies of organic solids determined by ultraviolet photoelectron spectroscopy. J Chem Soc Faraday Trans 2 Mol Chem Phys 77(9):1621–1633
Kronik LNeaton JB (2016) Excited-state properties of molecular solids from first principles. Annu Rev Phys Chem 67(1):587–616
Acknowledgments
The authors would like to acknowledge the support of the King Khalid University (KKU) for this research through a grant RCAMS/KKU/001-16 under the (Research Center for Advanced Materials Science) at King Khalid University, Kingdom of Saudi Arabia. Also, R. Ahmed is grateful to the Ministry of the Education/Universiti Teknologi Malaysia (UTM) for providing funding via project Q.J130000.2526.06H15 for the successful execution of this project.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1295 kb)
Rights and permissions
About this article
Cite this article
Chaudhry, A.R., Ahmed, R., Irfan, A. et al. Optoelectronic properties of naphtho[2, 1-b:6, 5-b′]difuran derivatives for photovoltaic application: a computational study. J Mol Model 22, 248 (2016). https://doi.org/10.1007/s00894-016-3121-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3121-y