Abstract
Bioethanol is one of the world’s most extensively produced biofuels. However, it is difficult to purify due to the formation of the ethanol–water azeotrope. Knowledge of the azeotrope structure at the molecular level can help to improve existing purification methods. In order to achieve a better understanding of this azeotrope structure, the characterization of (ethanol)5–water heterohexamers was carried out by analyzing the results of electronic structure calculations performed at the B3LYP/6-31+G(d) level. Hexamerization energies were found to range between −36.8 and −25.8 kcal/mol. Topological analysis of the electron density confirmed the existence of primary (OH…O) hydrogen bonds (HBs), secondary (CH…O) HBs, and H…H interactions in these clusters. Comparison with three different solvated alcohol systems featuring the same types of atom–atom interactions permitted the following order of stability to be determined: (methanol)5–water > (methanol)6 > (ethanol)5–water > (ethanol)6. These findings, together with accompanying geometric and spectroscopic analyses, show that similar cooperative effects exist among the primary HBs for structures with the same arrangement of primary HBs, regardless of the nature of the molecules involved. This result provides an indication that the molecular ratio can be considered to determine the unusual behavior of the ethanol–water system. The investigation also highlights the presence of several types of weak interaction in addition to primary HBs.

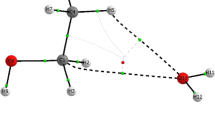
Water-ethanol clusters exhibit a variety of interaction types between their atoms, such as primary OH...O (blue), secondary CH...O (green) and H...H (yellow) interactions as revealed by Quantum Chemical Topology





Similar content being viewed by others
References
Lipnizki F (2010) Membrane process opportunities and challenges in the bioethanol industry. Desalination 250(3):1067–1069. doi:10.1016/j.desal.2009.09.109
Cardona CA, Sánchez ÓJ (2007) Fuel ethanol production: process design trends and integration opportunities. Bioresour Technol 98(12):2415–2457. doi:10.1016/j.biortech.2007.01.002
Dubey V, Pandey LK, Saxena C (2005) Pervaporative separation of ethanol/water azeotrope using a novel chitosan-impregnated bacterial cellulose membrane and chitosan–poly(vinyl alcohol) blends. J Membr Sci 251(1–2):131–136. doi:10.1016/j.memsci.2004.11.009
Budich M, Brunner G (2003) Supercritical fluid extraction of ethanol from aqueous solutions. J Supercrit Fluids 25(1):45–55. doi:10.1016/S0896-8446(02)00091-8
Wakisaka A, Matsuura K (2006) Microheterogeneity of ethanol–water binary mixtures observed at the cluster level. J Mol Liq 129(1–2):25–32. doi:10.1016/j.molliq.2006.08.010
Wakisaka A, Komatsu S, Usui Y (2001) Solute–solvent and solvent–solvent interactions evaluated through clusters isolated from solutions: preferential solvation in water–alcohol mixtures. J Mol Liq 90(1–3):175–184. doi:10.1016/S0167-7322(01)00120-9
Nishi N, Takahashi S, Matsumoto M, Tanaka A, Muraya K, Takamuku T, Yamaguchi T (1995) Hydrogen-bonded cluster formation and hydrophobic solute association in aqueous solutions of ethanol. J Phys Chem 99(1):462–468. doi:10.1021/j100001a068
Nishi N, Koga K, Ohshima C, Yamamoto K, Nagashima U, Nagami K (1988) Molecular association in ethanol–water mixtures studied by mass spectrometric analysis of clusters generated through adiabatic expansion of liquid jets. J Am Chem Soc 110(16):5246–5255. doi:10.1021/ja00224a002
Mijaković M, Kežić B, Zoranić L, Sokolić F, Asenbaum A, Pruner C, Wilhelm E, Perera A (2011) Ethanol–water mixtures: ultrasonics, Brillouin scattering and molecular dynamics. J Mol Liq 164(1–2):66–73. doi:10.1016/j.molliq.2011.06.009
Nishikawa K, Iijima T (1993) Small-angle X-ray scattering study of fluctuations in ethanol and water mixtures. J Phys Chem 97(41):10824–10828. doi:10.1021/j100143a049
Dixit S, Crain J, Poon WCK, Finney JL, Soper AK (2002) Molecular segregation observed in a concentrated alcohol–water solution. Nature 416(6883):829–832. doi:10.1038/416829a
Mizuno K, Miyashita Y, Shindo Y, Ogawa H (1995) NMR and FT-IR studies of hydrogen bonds in ethanol–water mixtures. J Phys Chem 99(10):3225–3228. doi:10.1021/j100010a037
Mashimo S, Umehara T, Redlin H (1991) Structures of water and primary alcohol studied by microwave dielectric analyses. J Chem Phys 95(9):6257–6260. doi:10.1063/1.461546
Nedić M, Wassermann TN, Xue Z, Zielke P, Suhm MA (2008) Raman spectroscopic evidence for the most stable water/ethanol dimer and for the negative mixing energy in cold water/ethanol trimers. Phys Chem Chem Phys 10(39):5953–5956. doi:10.1039/B811154E
Mó O, Yánez M, Elguero J (1992) Cooperative (nonpairwise) effects in water trimers: an ab initio molecular orbital study. J Chem Phys 97(9):6628–6638. doi:10.1063/1.463666
Hincapié G, Acelas N, Castano M, David J, Restrepo A (2010) Structural studies of the water hexamer. J Phys Chem A 114(29):7809–7814. doi:10.1021/jp103683m
Mejia SM, Orrego JF, Espinal JF, Fuentealba P, Mondragón F (2011) Exploration of the (ethanol)4–water heteropentamers potential energy surface by simulated annealing and ab initio molecular dynamics. Int J Quantum Chem 111(12):3080–3096. doi:10.1002/qua.22664
Mejia SM, Espinal JF, Mondragón F (2009) Cooperative effects on the structure and stability of (ethanol)3–water, (methanol)3–water heterotetramers and (ethanol)4, (methanol)4 tetramers. J Mol Struct (THEOCHEM) 901(1–3):186–193. doi:10.1016/j.theochem.2009.01.027
Mejia SM, Espinal JF, Restrepo A, Mondragon F (2007) Molecular interaction of (ethanol)2–water heterotrimers. J Phys Chem A 111(33):8250–8256. doi:10.1021/jp073168g
Fileti EE, Chaudhuri P, Canuto S (2004) Relative strength of hydrogen bond interaction in alcohol–water complexes. Chem Phys Lett 400(4–6):494–499. doi:10.1016/j.cplett.2004.10.149
Mejia SM, Mills MJL, Shaik MS, Mondragon F, Popelier PLA (2011) The dynamic behavior of a liquid ethanol–water mixture: a perspective from quantum chemical topology. Phys Chem Chem Phys 13(17):7821–7833. doi:10.1039/C0CP02869J
Popelier PLA (2005) Quantum chemical topology: on bonds and potentials. In: Wales DJ (ed) Intermolecular forces and clusters, vol 115. Springer, Berlin, pp 1–56. doi:10.1007/b101390
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University Press, Oxford
Laarhoven PJMV, Aarts EHL (1988) Simulated annealing: theory and applications. Mathematics and its applications. Kluwer, Dordrecht
Schlegel HB, Millam JM, Iyengar SS, Voth GA, Daniels AD, Scuseria GE, Frisch MJ (2001) Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. J Chem Phys 114(22):9758–9763. doi:10.1063/1.1372182
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven JT, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian, Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. 3. The role of exact exchange. J Chem Phys 98(7):5648–5652. doi:10.1063/1.464913
Lee CT, Yang WT, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electrondensity. Phys Lett B 37(2):785–789. doi:10.1103/PhysRevB.37.785
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab-initio calculation of vibrational absorption and circular-dichroism spectra using density-functional force-fields. J Phys Chem 98(45):11623–11627. doi:10.1021/j100096a001
Rozas I (2007) On the nature of hydrogen bonds: an overview on computational studies and a word about patterns. Phys Chem Chem Phys 9(22):2782–2790. doi:10.1039/b618225a
Johnson ER, DiLabio GA (2006) Structure and binding energies in van der Waals dimers: comparison between density functional theory and correlated ab initio methods. Chem Phys Lett 419(4–6):333–339. doi:10.1016/j.cplett.2005.11.099
Lozynski M, Rusinska-Roszak D, Mack H-G (1998) Hydrogen bonding and density functional calculations: the B3LYP approach as the shortest way to MP2 results. J Phys Chem A 102(17):2899–2903. doi:10.1021/jp973142x
Ciunik Z, Berski S, Latajka Z, Leszczynski J (1998) New aspects of weak C–H···π bonds: intermolecular interactions between alicyclic and aromatic rings in crystals of small compounds, peptides and proteins. J Mol Struct 442(1–3):125–134. doi:10.1016/S0022-2860(97)00288-3
Ramírez F, Hadad CZ, Guerra D, David J, Restrepo A (2011) Structural studies of the water pentamer. Chem Phys Lett 507(4–6):229–233. doi:10.1016/j.cplett.2011.03.084
Parthasarathi R, Subramanian V, Sathyamurthy N (2005) Hydrogen bonding in phenol, water, and phenol–water clusters. J Phys Chem A 109(5):843–850. doi:10.1021/jp046499r
Matta CF, Hernández-Trujillo J, Tang T-H, Bader RFW (2003) Hydrogen–hydrogen bonding: a stabilizing interaction in molecules and crystals. Chem Eur J 9(9):1940–1951. doi:10.1002/chem.200204626
Headgordon M, Pople JA, Frisch MJ (1988) MP2 energy evaluation by direct methods. Chem Phys Lett 153(6):503–506. doi:10.1016/0009-2614(88)85250-3
van Duijneveldt FB, van Duijneveldt-van de Rijdt JGCM, van Lenthe JH (1994) State of the art in counterpoise theory. Chem Rev 94(7):1873–1885. doi:10.1021/cr00031a007
Huyskens PL (1977) Factors governing the influence of a first hydrogen bond on the formation of a second one by the same molecule or ion. J Am Chem Soc 99(8):2578–2582. doi:10.1021/ja00450a028
Sauer J, Ugliengo P, Garrone E, Saunders VR (1994) Theoretical study of van der Waals complexes at surface sites in comparison with the experiment. Chem Rev 94(7):2095–2160. doi:10.1021/cr00031a014
Koch W, Holthausen MC (2001) A chemist’s guide to density functional theory, 2nd edn. Wiley-VCH, New York
Popelier PLA (1996) MORPHY, a program for an automated “atoms in molecules” analysis. Comput Phys Commun 93(2–3):212–240. doi:10.1016/0010-4655(95)00113-1
Rafat M, Devereux M, Popelier PLA (2005) Rendering of quantum topological atoms and bonds. J Mol Graph Model 24(2):111–120. doi:10.1016/j.jmgm.2005.05.004
Blender Foundation (2015) Blender—a 3D modeling and rendering package, 2.75a edn. Blender Foundation, Amsterdam
Popelier PLA (2000) Atoms in molecules: an introduction. Prentice Hall, Harlow
Liu K, Brown MG, Carter C, Saykally RJ, Gregory JK, Clary DC (1996) Characterization of a cage form of the water hexamer. Nature 381(6582):501–503. doi:10.1038/381501a0
Mayer I (1985) Bond orders and valences in the SCF theory: a comment. Theor Chim Acta (Berl) 67(4):315–322. doi:10.1007/bf00529303
Fulton RL, Perhacs P (1998) Sharing analysis of the behavior of electrons in some simple molecules. J Phys Chem A 102(45):8988–9000. doi:10.1021/jp982121f
Grabowski SJ (2011) What is the covalency of hydrogen bonding? Chem Rev 111(4):2597–2625. doi:10.1021/cr800346f
Koch U, Popelier PLA (1995) Characterization of C–H–O hydrogen bonds on the basis of the charge density. J Phys Chem 99(24):9747–9754. doi:10.1021/j100024a016
Mandal A, Prakash M, Kumar RM, Parthasarathi R, Subramanian V (2010) Ab initio and DFT studies on methanol–water clusters. J Phys Chem A 114(6):2250–2258. doi:10.1021/jp909397z
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122(45):11154–11161. doi:10.1021/ja0017864
Acknowledgments
The authors thank the Universidad de Antioquia for funding this project. SMM is grateful to the Research Vicerrectory at Pontificia Universidad Javeriana (project 6687). We express gratitude to Professor Paul Popelier of the University of Manchester for the use of the MORPHY program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 7938 kb)
Rights and permissions
About this article
Cite this article
Mejía, S.M., Espinal, J.F., Mills, M.J.L. et al. The role of OH…O and CH…O hydrogen bonds and H…H interactions in ethanol/methanol–water heterohexamers. J Mol Model 22, 181 (2016). https://doi.org/10.1007/s00894-016-3050-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3050-9