Abstract
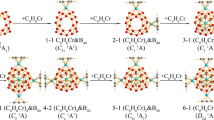
The recent discovery of perfect cage-like D 2d B40 − and D 2d B40 (all-boron fullerenes) has led to the emergence of a borospherene family. However, the geometrical and electronic structures of their cationic counterpart B40 +, previously detected in gas phase, remain unknown to date. Based on extensive first-principles theory calculations, we present herein the possibility of a perfect cage-like D 2d B40 + (1) (2A1) for the monocation, which turns out to be the global minimum of the system similar to B40 − and B40, adding a new member to the borospherene family. Molecular dynamics simulations indicate that D 2d B40 + (1) is dynamically stable at 300 K, whereas it starts to fluctuate at 500 K between the two lowest-lying isomers D 2d B40 + (1) (W) and C s B40 + (3) (M) in concerted W-X-M mechanisms via the transition state of C 1 B40 + (X), with forward (W → X → M) and backward (M → X → W) activation energies (Ea) of 14.6 and 6.9 kcal mol−1, respectively. The spectra from IR, Raman, and UV–vis analyses were simulated to facilitate future characterization of this important borospherene monocation.




Similar content being viewed by others
References
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York
Zhai HJ, Alexandrova AN, Birch KA, Boldyrev AI, Wang LS (2003) Hepta- and octacoordinated boron in molecular wheels of eight- and nine-atom boron clusters: observation and confirmation. Angew Chem Int Ed 42:6004–6008
Zhai HJ, Kiran B, Li J, Wang LS (2003) Hydrocarbon analogues of boron clusters —planarity, aromaticity and antiaromaticity. Nat Mater 2:827–833
Kiran B, Bulusu S, Zhai HJ, Yoo S, Zeng XC, Wang LS (2005) From the cover: planar-to-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes. Proc Natl Acad Sci USA 102:961–964
Huang W, Sergeeva AP, Zhai HJ, Averkiev BB, Wang LS, Boldyrev AI (2010) A concentric planar doubly π-aromatic B19 − cluster. Nat Chem 2:202–206
Oger E, Crawford NRM, Kelting R, Weis P, Kappes MM, Ahlrichs R (2007) Boron cluster cation: transition from planar to cylindrical structures. Angew Chem Int Ed 46:8503–8506
Li WL, Zhao YF, Hu HS, Li J, Wang LS (2014) [B30]−: a quasiplanar chiral boron cluster. Angew Chem Int Ed 53:5540–5545
Li WL, Chen Q, Tian WJ, Bai H, Zhao YF, Hu HS, Li J, Zhai HJ, Li SD, Wang LS (2014) The B35 cluster with a double-hexagonal vacancy: a new and more flexible structural motif for borophene. J Am Chem Soc 136:12257–12260
Piazza ZA, Hu HS, Li WL, Zhao YF, Li J, Wang LS (2014) Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat Commun 5:3113(6)
Chen Q, Wei GF, Tian WJ, Bai H, Liu ZP, Zhai HJ, Li SD (2014) Quasi-planar aromatic B36 and B36 − clusters: all-boron analogues of coronene. Phys Chem Chem Phys 16:18282–18287
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS (2006) All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord Chem Rev 250:2811–2866
Romanescu C, Galeev TR, Li WL, Boldyrev AI, Wang LS (2013) Transition-Metal-Centered monocyclic boron wheel clusters (M©Bn):a new class of aromatic borometallic compounds. Acc Chem Res 46:350–358
Sergeeva AP, Popov IA, Piazza ZA, Li WL, Romanescu C, Wang LS, Boldyrev AI (2014) Understanding boron through size-selected clusters: structure, chemical bonding, and fluxionality. Acc Chem Res 47:1349–1358
Zhai HJ, Zhao YF, Li WL, Chen Q, Bai H, Hu HS, Piazza ZA, Tian WJ, Lu HG, Wu YB, Mu YW, Wei GF, Liu ZP, Li J, Li SD, Wang LS (2014) Observation of an all-boron fullerene. Nat Chem 6:727–731
Bai H, Chen Q, Zhai HJ, Li SD (2015) Endohedral and exohedral metalloborospherenes: M@B40 (M = Ca, Sr) and M&B40 (M = Be, Mg). Angew Chem Int Ed 54:941–945
He RX, Zeng XC (2015) Electronic structures and electronic spectra of all-boron fullerene B40. Chem Commun 51:3185–3188
Chen Q, Zhang SY, Bai H, Tian WJ, Gao T, Li HR, Miao CQ, Mu YW, Lu HG, Zhai HJ, Li SD (2015) Cage-like B41 + and B42 2+: new chiral members of the borospherene family. Angew Chem Int Ed 54:8160–8164
Chen Q, Li WL, Zhao YF, Zhang SY, Hu HS, Bai H, Li HR, Tian WJ, Lu HG, Zhai HJ, Li SD, Wang LS (2015) Experimental and theoretical evidence of an axially chiral borospherene. ACS Nano 9:754–760
Chen Q, Li HR, Miao CQ, Wang YJ, Lu HG, Mu YW, Ren GM, Zhai HJ, Li SD (2016) Endohedral Ca@B38: stabilization of a B38 2− borospherene dianion by metal encapsulation. Phys Chem Chem Phys. doi:10.1039/c5cp06169e
Tian WJ, Chen Q, Li HR, Yan M, Mu YW, Lu HG, Zhai HJ, Li SD (2016) Saturn-like charge-transfer complexes Li4&B36, Li5&B36 +, and Li6&B36 2+: exohedral metalloborospherenes with a perfect cage-like B36 4− core. Phys Chem Chem Phys. doi:10.1039/C6CP01279E
Tai TB, Nguyen MT (2016) A new chiral boron cluster B44 containing nonagonal holes. Chem Commun 52:1653–1656
Wang YJ, Zhao YF, Li WL, Jian T, Chen Q, You XR, Ou T, Zhao XY, Zhai HJ, Li SD, Li J, Wang LS (2016) Observation and characterization of the smallest borospherene, B28 − and B28. J Chem Phys 144:064307
Mannix AJ, Zhou XF, Kiraly B, Wood JD, Alducin D, Myers BD, Liu XL, Fisher BL, Santiago U, Guest JR, Yacaman MJ, Ponce A, Oganov AR, Hersam MC, Guisinger NP (2015) Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350:1513–1516
Feng BJ, Zhang J, Zhong Q, Li WB, Li S, Li H, Cheng P, Meng S, Chen L, Wu KH (2016) Experimental realization of two-dimensional boron sheets. Arxiv:1512.05029
Placa SJL, Roland PA, Wynne JJ (1992) Boron clusters (Bn, n = 2-52) produced by laser ablation of hexagonal boron nitride. Chem Phys Lett 190:163–168
De S, Willand A, Amsler M, Pochet P, Genovese L, Goedecker S (2011) Energy landscape of fullerene materials: a comparison of boron to boron nitride and carbon. Phys Rev Lett 106:225502
Goedecker S, Hellmann W, Lenosky T (2005) Global minimum determination of the Born-Oppenheimer surface within density functional theory. Phys Rev Lett 95:055501
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Tao JM, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave-functions. J Chem Phys 72:650–654
Čížek J (1969) On the use of the cluster expansion and the technique of diagrams in calculations of correlation effects in atoms and molecules. Adv Chem Phys 14:35–89
Purvis GD III, Bartlett RJ (1982) A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J Chem Phys 76:1910–1918
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem Phys Lett 157:479–483
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Errata: Generalized gradient approximation made simple. Phys Rev Lett 78:1396
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Perdew JP, Ziesche EP, Eschrig H (1991) Electronic Structure of Solids ‘91. Akademie, Berlin
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2013) Gaussian 09, revision D.01. Gaussian, Inc, Wallingford
Werner HJ, Knowles PJ, Knizia G, Manby FR, Schütz M, Celani P, Korona T, Lindh R, Mitrushenkov A, Rauhut G, et al (2012) MOLPRO, version 2012.1.
VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) Quickstep:fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput Phys Commun 167:103–128
Bauernschmitt R, Ahlrichs R (1996) Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem Phys Lett 256:454–464
Gao TT, Chen Q, Mu YW, Lu HG, Li SD (2016) “W-X-M” transformation in isomerization of B39 − borospherenes. J Chem Phys (in press)
Martínez-Guajardo G, Cabellos JL, Díaz-Celaya A, Pan S, Islas R, Chattaraj PK, Heine T, Merino G (2015) Dynamical behavior of borospherene: a nanobubble. Sci Rep 5:11287. doi:10.1038/srep11287
Wang GJ, Zhou MF, Goettel JT, Schrobilgen GJ, Su J, Li J, Schlöder T, Riedel S (2014) Identification of an iridium-containing compound with a formal oxidation state of IX. Nature 514:475–477
Ciuparu D, Klie RF, Zhu YM, Pfefferle L (2004) Synthesis of pure boron single-wall nanotubes. J Phys Chem B 108:3967–3969
Acknowledgment
This work was supported financially by the National Natural Science Foundation of China (21373130, 21573138, and 21473106).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2049 kb)
Rights and permissions
About this article
Cite this article
Li, HR., Chen, Q., Tian, XX. et al. Cage-like B40 +: a perfect borospherene monocation. J Mol Model 22, 124 (2016). https://doi.org/10.1007/s00894-016-2980-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-2980-6