Abstract
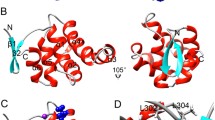
Death receptor 5 (DR5)-induced apoptosis that prioritizes the death of tumor cells has been proposed as one of the promising cancer therapies. In this process, oligomerized DR5 death domain (DD) binding to Fas-associated death domain (FADD) leads to FADD activating caspase-8, which marks the formation of the death-inducing signaling complex (DISC) that initiates apoptosis. DR5 DD mutations found in cancer cells have been suggested to play an important pathological role, the mechanism through which those mutants prevent the DR5-activated DISC formation is not clear yet. This study sought to provide structural and molecular insight for the roles of four selected DR5 DD mutations (E355K, E367K, K415N, and L363F) in the oligomerization of DR5 DD–FADD complex during the DISC formation. Results from the molecular dynamics simulations show that the simulated mutants induce conformational, dynamical motions and interactions changes in the DR5 DD–FADD tetramer complex, including changes in a protein’s backbone flexibility, less exposure of FADD DED’s caspase-8 binding site, reduced H-bonding and hydrophobic contacts at the DR5 DD–FADD DD binding, altered distribution of the electrostatic potentials and correlated motions of residues, and reduced binding affinity of DR5 DD binding to FADD. This study provides structural and molecular insight for the influence of DR5 DD mutations on oligomerization of DR5 DD–FADD complex, which is expected to foster understanding of the DR5 DD mutants’ resistance mechanism against DR5-activated DISC formation.





Similar content being viewed by others
Abbreviations
- DD:
-
Death domain
- DED:
-
Death effector domain
- DISC:
-
Death-inducing signaling complex
- DR5:
-
Death receptor 5
- FADD:
-
Fas-associated protein with death domain
- TNFR:
-
Tumor necrosis factor receptor
- FasL:
-
Fas ligand
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- MD:
-
Molecular dynamics
- NPT:
-
Constant number-pressure-temperature
- NVT:
-
Constant number-volume-temperature
- DCCM:
-
Dynamical cross correlation maps
- MM-PBSA:
-
The molecular mechanics/Poisson–Boltzmann surface area
References
Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11:255–260
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10:26–35
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241
Ashkenazi A (2002) Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2:420–430
Thorburn A (2004) Death receptor-induced cell killing. Cell Signal 16:139–144
Kantari C, Walczak H (2011) Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta 1813:558–563
Stuckey DW, Shah K (2013) TRAIL on trial: preclinical advances in cancer therapy. Trends Mol Med 19:685–694
Ozoren N, El-Deiry WS (2002) Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia 4:551–557
Khosravi-Far R, Esposti MD (2004) Death receptor signals to mitochondria. Cancer Biol Ther 3:1051–1057
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease, Cold Spring Harb Perspect Biol 5, a008656
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52:1–8
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61
Clemens MJ, van Venrooij WJ, van de Putte LB (2000) Apoptosis and autoimmunity. Cell Death Differ 7:131–133
Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21:485–495
Gasparian ME, Bychkov ML, Yagolovich AV, Dolgikh DA, Kirpichnikov MP (2015) Mutations Enhancing Selectivity of Antitumor Cytokine TRAIL to DR5 Receptor Increase Its Cytotoxicity against Tumor Cells. Biochemistry (Mosc) 80:1080–1091
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5:157–163
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP, Zhou T (2001) Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 7:954–960
Jin H, Yang R, Fong S, Totpal K, Lawrence D, Zheng Z, Ross J, Koeppen H, Schwall R, Ashkenazi A (2004) Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res 64:4900–4905
Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A (2005) Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem 280:2205–2212
Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol Off J Am Soc Clin Oncol 26:3621–3630
Camidge DR (2008) Apomab: an agonist monoclonal antibody directed against Death Receptor 5/TRAIL-Receptor 2 for use in the treatment of solid tumors. Expert Opin Biol Ther 8:1167–1176
Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, Takeda K, Smyth MJ, Murphy WJ, Sayers TJ (2008) Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst 100:649–662
Rosevear HM, Lightfoot AJ, Griffith TS (2010) Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer. Curr Opin Investig Drugs 11:688–698
Sharma S, de Vries EG, Infante JR, Oldenhuis CN, Gietema JA, Yang L, Bilic S, Parker K, Goldbrunner M, Scott JW, Burris HA 3rd (2014) Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Investig New Drugs 32:135–144
Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5:876–885
Guo Y, Chen C, Zheng Y, Zhang J, Tao X, Liu S, Zheng D, Liu Y (2005) A novel anti-human DR5 monoclonal antibody with tumoricidal activity induces caspase-dependent and caspase-independent cell death. J Biol Chem 280:41940–41952
Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A (2009) TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev 35:280–288
Pavet V, Beyrath J, Pardin C, Morizot A, Lechner MC, Briand JP, Wendland M, Maison W, Fournel S, Micheau O, Guichard G, Gronemeyer H (2010) Multivalent DR5 peptides activate the TRAIL death pathway and exert tumoricidal activity. Cancer Res 70:1101–1110
Sakai T (2011) “Molecular-targeting prevention” of cancer. The theory and its possibilities. Nihon eiseigaku zasshi Jpn J Hyg 66:3–12
Mahalingam D, Oldenhuis CN, Szegezdi E, Giles FJ, de Vries EG, de Jong S, Nawrocki ST (2011) Targeting TRAIL towards the clinic. Curr Drug Targets 12:2079–2090
Lee SH, Shin MS, Kim HS, Lee HK, Park WS, Kim SY, Lee JH, Han SY, Park JY, Oh RR, Jang JJ, Han JY, Lee JY, Yoo NJ (1999) Alterations of the DR5/TRAIL receptor 2 gene in non-small cell lung cancers. Cancer Res 59:5683–5686
Shin MS, Kim HS, Lee SH, Park WS, Kim SY, Park JY, Lee JH, Lee SK, Lee SN, Jung SS, Han JY, Kim H, Lee JY, Yoo NJ (2001) Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res 61:4942–4946
Park WS, Lee JH, Shin MS, Park JY, Kim HS, Kim YS, Park CH, Lee SK, Lee SH, Lee SN, Kim H, Yoo NJ, Lee JY (2001) Inactivating mutations of KILLER/DR5 gene in gastric cancers. Gastroenterology 121:1219–1225
El-Naggar AK, Coombes MM, Batsakis JG, Hong WK, Goepfert H, Kagan J (1998) Localization of chromosome 8p regions involved in early tumorigenesis of oral and laryngeal squamous carcinoma. Oncogene 16:2983–2987
Yustein AS, Harper JC, Petroni GR, Cummings OW, Moskaluk CA, Powell SM (1999) Allelotype of gastric adenocarcinoma. Cancer Res 59:1437–1441
Emi M, Fujiwara Y, Nakajima T, Tsuchiya E, Tsuda H, Hirohashi S, Maeda Y, Tsuruta K, Miyaki M, Nakamura Y (1992) Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res 52:5368–5372
Kagan J, Stein J, Babaian RJ, Joe YS, Pisters LL, Glassman AB, von Eschenbach AC, Troncoso P (1995) Homozygous deletions at 8p22 and 8p21 in prostate cancer implicate these regions as the sites for candidate tumor suppressor genes. Oncogene 11:2121–2126
Wistuba II, Behrens C, Virmani AK, Milchgrub S, Syed S, Lam S, Mackay B, Minna JD, Gazdar AF (1999) Allelic losses at chromosome 8p21-23 are early and frequent events in the pathogenesis of lung cancer. Cancer Res 59:1973–1979
Lerebours F, Olschwang S, Thuille B, Schmitz A, Fouchet P, Buecher B, Martinet N, Galateau F, Thomas G (1999) Fine deletion mapping of chromosome 8p in non-small-cell lung carcinoma. Int J Cancer 81:854–858
Bin L, Thorburn J, Thomas LR, Clark PE, Humphreys R, Thorburn A (2007) Tumor-derived mutations in the TRAIL receptor DR5 inhibit TRAIL signaling through the DR4 receptor by competing for ligand binding. J Biol Chem 282:28189–28194
Sun SY (2011) Understanding the role of the death receptor 5/FADD/caspase-8 death signaling in cancer metastasis. Mol Cell Pharmacol 3:31–34
McDonald ER 3rd, Chui PC, Martelli PF, Dicker DT, El-Deiry WS (2001) Death domain mutagenesis of KILLER/DR5 reveals residues critical for apoptotic signaling. J Biol Chem 276:14939–14945
Cha SS, Sung BJ, Kim YA, Song YL, Kim HJ, Kim S, Lee MS, Oh BH (2000) Crystal structure of TRAIL-DR5 complex identifies a critical role of the unique frame insertion in conferring recognition specificity. J Biol Chem 275:31171–31177
Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O’Connell M, Kelley RF, Ashkenazi A, de Vos AM (1999) Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell 4:563–571
Mongkolsapaya J, Grimes JM, Chen N, Xu XN, Stuart DI, Jones EY, Screaton GR (1999) Structure of the TRAIL-DR5 complex reveals mechanisms conferring specificity in apoptotic initiation. Nat Struct Biol 6:1048–1053
Hymowitz SG, O’Connell MP, Ultsch MH, Hurst A, Totpal K, Ashkenazi A, de Vos AM, Kelley RF (2000) A unique zinc-binding site revealed by a high-resolution X-ray structure of homotrimeric Apo2L/TRAIL. Biochemistry 39:633–640
Banner DW, D’Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W (1993) Crystal structure of the soluble human 55-kD TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 73:431–445
Blanchard H, Kodandapani L, Mittl PR, Marco SD, Krebs JF, Wu JC, Tomaselli KJ, Grutter MG (1999) The three-dimensional structure of caspase-8: an initiator enzyme in apoptosis. Structure 7:1125–1133
Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS (2003) A unified model for apical caspase activation. Mol Cell 11:529–541
Donepudi M, Mac Sweeney A, Briand C, Grutter MG (2003) Insights into the regulatory mechanism for caspase-8 activation. Mol Cell 11:543–549
Watt W, Koeplinger KA, Mildner AM, Heinrikson RL, Tomasselli AG, Watenpaugh KD (1999) The atomic-resolution structure of human caspase-8, a key activator of apoptosis. Structure 7:1135–1143
Valley CC, Lewis AK, Mudaliar DJ, Perlmutter JD, Braun AR, Karim CB, Thomas DD, Brody JR, Sachs JN (2012) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces death receptor 5 networks that are highly organized. J Biol Chem 287:21265–21278
Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E, Robinson H, Salvesen GS, Schwarzenbacher R, Riedl SJ (2009) The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature 457:1019–1022
Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW (1996) NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384:638–641
Yan Q, McDonald JM, Zhou T, Song Y (2013) Structural insight for the roles of fas death domain binding to FADD and oligomerization degree of the Fas-FADD complex in the death-inducing signaling complex formation: a computational study. Proteins 81:377–385
Carrington PE, Sandu C, Wei Y, Hill JM, Morisawa G, Huang T, Gavathiotis E, Wei Y, Werner MH (2006) The structure of FADD and its mode of interaction with procaspase-8. Mol Cell 22:599–610
Ozoren N, El-Deiry WS (2003) Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol 13:135–147
Arai T, Akiyama Y, Okabe S, Saito K, Iwai T, Yuasa Y (1998) Genomic organization and mutation analyses of the DR5/TRAIL receptor 2 gene in colorectal carcinomas. Cancer Lett 133:197–204
Sigrist CJ, De Castro E, Langendijk-Genevaux PS, Le Saux V, Bairoch A, Hulo N (2005) ProRule: a new database containing functional and structural information on PROSITE profiles. Bioinformatics 21:4060–4066
Notredame C, Higgins DG, Heringa J (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302:205–217
Chang JM, Di Tommaso P, Notredame C (2014) TCS: a new multiple sequence alignment reliability measure to estimate alignment accuracy and improve phylogenetic tree reconstruction. Mol Biol Evol 31:1625–1637
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815–818
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519
Vriend G (1990) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8(52–56):29
Eisenberg D, Luthy R, Bowie JU (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 35:W407–W410
Puklin-Faucher E, Gao M, Schulten K, Vogel V (2006) How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J Cell Biol 175:349–360
Pan D, Song Y (2010) Role of altered sialylation of the I-like domain of beta1 integrin in the binding of fibronectin to beta1 integrin: thermodynamics and conformational analyses. Biophys J 99:208–217
Case DA, Cheatham TE 3rd, Darden T, Gohlke H, Luo R, Merz KM Jr, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926
Wang L, Murphy-Ullrich JE, Song Y (2014) Molecular insight into the effect of lipid bilayer environments on thrombospondin-1 and calreticulin interactions. Biochemistry 53:6309–6322
Yan Q, Murphy-Ullrich JE, Song Y (2011) Molecular and structural insight into the role of key residues of thrombospondin-1 and calreticulin in thrombospondin-1-calreticulin binding. Biochemistry 50:566–573
Yan Q, Murphy-Ullrich JE, Song Y (2010) Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry 49:3685–3694
Suever JD, Chen Y, McDonald JM, Song Y (2008) Conformation and free energy analyses of the complex of calcium-bound calmodulin and the Fas death domain. Biophys J 95:5913–5921
Liu Y, Pan D, Bellis SL, Song Y (2008) Effect of altered glycosylation on the structure of the I-like domain of beta1 integrin: a molecular dynamics study. Proteins 73:989–1000
Lee SJ, Song Y, Baker NA (2008) Molecular dynamics simulations of asymmetric NaCl and KCl solutions separated by phosphatidylcholine bilayers: potential drops and structural changes induced by strong Na + −lipid interactions and finite size effects. Biophys J 94:3565–3576
Song Y, Guallar V, Baker NA (2005) Molecular dynamics simulations of salicylate effects on the micro- and mesoscopic properties of a dipalmitoylphosphatidylcholine bilayer. Biochemistry 44:13425–13438
Miller BR, McGee TD, Swails JM, Homeyer N, Gohlke H, Roitberg AE (2012) MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theory Comput 8:3314–3321
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449–461
Pan D, Yan Q, Chen Y, McDonald JM, Song Y (2011) Trifluoperazine regulation of calmodulin binding to Fas: a computational study. Proteins 79:2543–2556
Humphrey W, Dalke A, Schulten K (1996) VMD - visual molecular dynamics. J Mol Graph 14:33–38
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A 98:10037–10041
Acknowledgments
The authors thank Alabama Supercomputer Authority and UAB Cheaha cluster for providing computing time.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by an NIH K25 award (5K25CA140791) to Y. H. Song.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 29777 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Song, Y. Structural Insight for Roles of DR5 Death Domain Mutations on Oligomerization of DR5 Death Domain–FADD Complex in the Death-Inducing Signaling Complex Formation: A Computational Study. J Mol Model 22, 89 (2016). https://doi.org/10.1007/s00894-016-2941-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-2941-0