Abstract
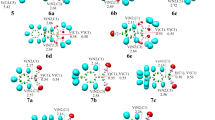
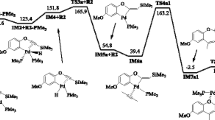
The Wittig reaction of cyclopropanone, cyclobutanone and cyclopentanone with phosphorus ylide (Me3P = CH2) in gas phase was investigated computationally at B3LYP/6-31G** level of theory. In the Wittig reaction of cyclic ketones, two transition states (TS1 and TS2), corresponding to formation and decomposition of oxaphosphetane (OP) were located and investigated. Two loosely bound intermediates, a reactant complex (RC) and a product complex (PC) were also found. In the reaction of cyclopropanone, cyclobutanone and cyclopentanone, two oxaphosphetanes (OP1 and OP2) were predicted. OP1 initially formed was converted into OP2 by pseudorotation. In contrast to the reactions with cyclobutanone and cyclopentanone, an early TS1 was found in the reaction of cyclopropanone. The order of first activation energy barrier relative to reactant total energy was found to be cyclopropanone (−4.97 kcal mol−1) < cyclobutanone (0.30 kcal mol−1) < cyclopentanone (3.60 kcal mol−1).

The Wittig reaction of monocyclic ketones with phosphorus ylide Me3PCH2.






Similar content being viewed by others
Notes
References
Wittig G, Schollkopf U (1954) Über triphenyl-phosphin-methylene alsolefinbildendereagenzien (I. Mitteil). Chem Ber 87:1318–1330. doi:10.1002/cber.19540870919
Wittig G (1964) Variationen zu einem thema von staudinger; einbeitragzur geschichte der phosphororganischen carbonyl-olefinierung. Pure Appl Chem 9:245–254. doi:10.1351/pac196409020245
Johnson AW (1993) Ylides and imines of phosphorus. Wiley, New York
Maryanoff BE, Reitz AB (1989) The Wittig olefination reaction and modifications involving phosphoryl stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem Rev 89:863–927. doi:10.1021/cr00094a007
Vedejs E, Peterson MJ (1994) Stereochemistry and mechanism of the Wittig reaction. In: Eliel EL, Wilen SH (eds) Topics in stereochemistry, vol 21. Wiley, Hoboken, pp 1–157. doi: 10.1002/9780470147306.ch1
Vedejs E, Snoble KAJ (1973) Direct observation of oxaphosphetanes from typical Wittig reactions. J Am Chem Soc 95:5778–5780. doi:10.1021/ja00798a066
Vedejs E, Meier GP, Snoble KAJ (1981) Low-temperature characterization of the intermediates in the Wittig reaction. J Am Chem Soc 103:2823–2831. doi:10.1021/ja00400a055
Reitz AB, Mutter MS, Maryanoff BE (1984) Observation of cis and trans oxaphosphetanes in the Wittig reaction by high-field 31P NMR spectroscopy. J Am Chem Soc 106:1873–1875. doi:10.1021/ja00318a073
Maryanoff BE, Reitz AB, Mutter MS, Inners RR, Almond HR Jr, Whittle RR, Olofson RA (1986) Stereochemistry and mechanism of the Wittig reaction. Diastereomeric reaction intermediates and analysis of the reaction course. J Am Chem Soc 108:7664–7678. doi:10.1021/ja00284a034
Vedejs E, Marth CF (1989) Oxaphosphetane pseudorotation: rates and mechanistic significance in the Wittig reaction. J Am Chem Soc 111:1519–1520. doi:10.1021/ja00186a068
Bangerter F, Karpf M, Meier LA, Rys P, Skabal P (1998) Observation of pseudorotamers of two unconstrained Wittig intermediates, (3RS,4SR)- and (3RS,4RS)-4-cyclohexyl-2-ethyl-3,4-dimethyl-2,2-diphenyl-1,2λ5-oxaphosphetane, by dynamic 31P NMR spectroscopy: line-shape analyses, conformations, and decomposition kinetics. J Am Chem Soc 120:10653–10659. doi:10.1021/ja974332o
Appel M, Blaurock S, Berger S (2002) A Wittig reaction with 2‐furyl substituents at the phosphorus atom: improved (Z) selectivity and isolation of a stable oxaphosphetane intermediate. Eur J Org Chem 2002:1143–1148. doi:10.1002/1099-0690(200204)2002:7<1143::AID-EJOC1143>3.0.CO;2-G
Vedejs E, Fleck TJ (1989) Kinetic (not equilibrium) factors are dominant in Wittig reactions of conjugated ylides. J Am Chem Soc 111:5861–5871. doi:10.1021/ja00197a055
Vedejs E, Peterson MJ (1996) In: Snieckus V (ed) In advances in carbanion chemistry. Jai Press Inc, Greenwich, pp 1–85
Olah GA, Krishnamurthy VV (1982) Unusual solvent effects in the Wittig reaction of some ketones indicating initial one-electron transfer. J Am Chem Soc 104:3987–3990. doi:10.1021/ja00378a035
Yamataka H, Nagareda K, Takatsuka T, Ando K, Hanafusa T, Nagase S (1993) Distinction between polar and electron-transfer routes. A mechanistic study on the Wittig reactions of nonstabilizedylides. J Am Chem Soc 115:8570–8576. doi:10.1021/ja00072a008
Vedejs E, Marth CF (1990) Mechanism of Wittig reaction: evidence against betaine intermediates. J Am Chem Soc 112:3905–3909. doi:10.1021/ja00166a026
Witschard G, Griffin CE (1964) The Wittig reaction with five-and six-membered cyclic ketones and their benzylidene derivatives. J Org Chem 29:2335–2340. doi:10.1021/jo01031a057
Wu J, Li D, Wu H, Sun L, Dai W-M (2006) Microwave-assisted regioselective olefinations of cyclic mono- and di-ketones with a stabilized phosphorus ylide. Tetrahedron 62:4643–4650. doi:10.1016/j.tet.2005.12.060
Wu J, Wu H, Wei S, Dai W-M (2004) Highly regioselective Wittig reactions of cyclic ketones with a stabilized phosphorus ylide under controlled microwave heating. Tetrahedron Lett 45:4401–4404. doi:10.1016/j.tetlet.2004.03.198
Ghosh A, Chakraborty I, Adarsh NN, Lahiri S (2010) Wittig-selectivity in mixed ketones: exploring 1, 3-interaction and enolization. Tetrahedron 66:164–171. doi:10.1016/j.tet.2009.11.006
Mari F, Lahti PM, McEwen WE (1990) Molecular modeling oxaphosphetane intermediates of Wittig olefination reactions. Heteroat Chem 1:255–259. doi:10.1002/hc.520010311
Mari F, Lahti PM, McEwen WE (1991) Molecular modeling of the Wittig olefination reaction: part 2: a molecular orbital approach at the MNDO-PM3 level. Heteroat Chem 2:265–276. doi:10.1002/hc.520020208
Mari F, Lahti PM, McEwen WE (1992) A theoretical study of the Wittig olefination reaction: MNDO-PM3 treatment of the Wittig half-reaction of unstabilizedylides with aldehydes. J Am Chem Soc 114:813–821. doi:10.1021/ja00029a002
Yamataka H, Hanafusa T, Nagase S, Kurakake T (1991) Theoretical study on the transition state of oxaphosphetane formation between ethylidenetriphenylphosphorane and acetaldehyde. Heteroat Chem 2:465–468. doi:10.1002/hc.520020407
Holler R, Lischka H (1980) A theoretical investigation on the model Wittig reaction PH3CH2 + CH2O → PH3O + C2H4. J Am Chem Soc 102:4632–4635. doi:10.1021/ja00534a011
Volatron F, Eisenstein O (1984) Theoretical study of the reactivity of phosphonium and sulfoniumylides with carbonyl groups. J Am Chem Soc 106:6117–6119. doi:10.1021/ja00332a081
Volatron F, Eisenstein O (1987) Wittig vs Corey-Chaykovsky reaction. A theoretical study of the reactivity of phosphoniummethylide and sulfoniummethylide with formaldehyde. J Am Chem Soc 109:1–14. doi:10.1021/ja00235a001
Naito T, Nagase S, Yamataka H (1994) Theoretical study of the structure and reactivity of ylides of N, P, As, Sb, and Bi. J Am Chem Soc 116:10080–10088. doi:10.1021/ja00101a029
Restrepo-Cossio AA, Gonzalez CA, Mari F (1998) Comparative Ab initio treatment (hartree-fock, density functional theory, MP2, and quadratic configuration interactions) of the cycloaddition of phosphorus ylides with formaldehyde in the gas phase. J Phys Chem A 102:6993–7000. doi:10.1021/jp981085h
Lu WC, Wong NB, Zhang RQ (2002) Theoretical study on the substituent effect of a Wittig reaction. Theor Chem Acc 107:206–210. doi:10.1007/s00214-001-0320-z
Robiette R, Richardson J, Aggarwal VK, Harvey JN (2006) Reactivity and selectivity in the Wittig reaction: a computational study. J Am Chem Soc 128:2394–2409. doi:10.1021/ja056650q
Yamataka H, Nagase S (1998) Theoretical calculations on the Wittig reaction revisited. J Am Chem Soc 120:7530–7536. doi:10.1021/ja974237f
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian Inc, Wallingford
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theoret Chim Acta (Berl) 28:213–222
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. doi:10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163. doi:10.1021/j100717a029
Restrepo-Cossio AA, Cano H, Gonzalez CA, Mari F (1997) Molecular modeling of the Wittig olefination reaction: MNDO-PM3 and Ab initio treatment of the Wittig reaction of unstabilized, semistabilized and stabilized ylides with acetaldehyde. Heteroat Chem 8:557–569. doi:10.1002/(SICI)1098-1071(1997)8:6<557::AID-HC16>3.0.CO;2-Q
Dennington R, Keith T, Millam J (2009) GaussView, Version 5. Semichem Inc, Shawnee Mission
Bach RD, Dmitrenko O (2006) The effect of carbonyl substitution on the strain energy of small ring compounds and their six-member ring reference compounds. J Am Chem Soc 128:4598–4611. doi:10.1021/ja055086g
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338. doi:10.1021/ja01607a027
Acknowledgments
One of the authors (N.J.) is thankful to the council of scientific and industrial research (CSIR), Government of India for the award of a fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Total electronic energy, enthalpy and Gibbs free energy (in Hartree) and the Cartesian coordinates of all optimized structures with imaginary frequencies of transition states (TS) are given in the supporting information. (DOCX 524 kb)
Rights and permissions
About this article
Cite this article
Jarwal, N., Thankachan, P.P. Theoretical study of the Wittig reaction of cyclic ketones with phosphorus ylide. J Mol Model 21, 87 (2015). https://doi.org/10.1007/s00894-015-2571-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2571-y