Abstract
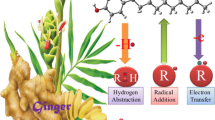
The anti-oxidant action of lycopene as a hydroxyl radical scavenger through hydrogen abstraction and addition reaction mechanisms has been investigated. Geometries of seven different conformations of lycopene were optimized employing density functional theory in gas phase which was followed by treatment of their solvation in aqueous media. Thus the all-trans conformation of lycopene was found to be most stable in both gas phase and aqueous media. Four overlapping fragments of all-trans lycopene were considered for calculations of Gibbs barrier energies and rate constants. It is found that several hydrogen atoms can be abstracted from lycopene by a hydroxyl radical barrierlessly. Further, it is shown that addition of an OH radical can also take place to each of the various carbon atoms of lycopene with fairly low barrier energies. Thus lycopene is shown to be an effective anti-oxidant.

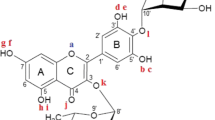
Structure of all-trans lycopene





Similar content being viewed by others
References
Liu Z-Q (2010) Chem Rev 110:5675–5691
Halliwell B (1996) Ann Rev Nutr 16:33–50
Burrows CJ, Muller JG (1998) Chem Rev 98:1109–1151
Hussain SP, Hofseth LJ, Harris CC (2003) Nature 3:276–285
Bruner SD, Norman DPG, Verdine GC (2000) Nature 403:859–866
Jena NR, Mishra PC, Suhai S (2009) J Phys Chem B 113:5633–5644
Yadav A, Mishra PC (2012) Chem Phys 405:76–88
Mishra PC (1989) J Mol Struct 195:201–211
McCall MR, Frei B (1999) Free Rad Biol Med 26:1034–1053
Prasad AK, Mishra PC (2013) J Phy Org Chem. doi:10.1002/poc.3200
Shukla MK, Mishra PC (1996) J Mol Struct 377:247–259
Kim D-O, Lee CY (2004) Crit Rev Food Sci Nutr 44:253–273
Chen H-JC, Wu S-B, Chang C-M (2003) Arch Biochem Biophys 415:109–116
Krinsky NI, Johnson EJ (2005) Mol Asp Med 26:459–516
Halliwell B, Murcia MA, Chirico S, Aruoma OI (1995) Crit Rev Food Sci Nutr 35:7–20
Sies H, Stahl W (1995) Am J Clin Nutr 62:1315S–1321S
Feri B (1994) Natural Anti-oxidants in Human Health and Disease, Academic Press, San Diego
Krinsky NI (2001) Nutrition 17:815–817
Galano A, Francisco-Marquez M (2009) J Phys Chem B 113:11338–11345
Stahl W, Sies H (2005) Biochim Biophys Acta 1740:101–107
Clinton SK (1998) Nutr Rev 56:35–51
Martinez-Valverde I, Periago MJ, Provan G, Chesson A (2002) J Sci Food Agric 82:323–330
Guo W-H, Tu C-Y, Hu C-H (2008) J Phys Chem B 112:12158–12167
Stahl W, Sies H (1996) Arch Biochem Biophy 336(1):1-9
Collins JK, Veazie PP, Roberts W (2006) Hortic Sci 41:1135–1144
Britton G (1995) FASEB J 9:1551–1558
Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA (1997) FEBS Lett 418:91–97
Johnson EJ (2002) Nutr Clin Care 5:56–65
Porrini M, Riso P (2000) J Nutr 130:189–192
Palozza P, Simone RE, Catalano A, Mele MC (2011) Cancers 3:2333–2357
Graham DL, Carail M, Caris-Veyrat C, Lowe GM (2010) Food Chem Toxicol 48:2413–2420
Rao AV, Agarwal S (1998) Nutr Res 189:713–721
Kucuk O, Sarkar FH, Sakr W et al (2001) Cancer Epidemiol Biomarkers Prev 10:861–868
Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A (2012) Ann Nutr Metab 61:126–134
Qu M, Zhou Z, Chen C, Li M, Pei L, Chu F, Yang J, Wang Y, Li L, Liu C, Zhang L, Zhang G, Yu Z, Wang D (2011) Neurochem Int 59:1095–1103
Helzlsouer KJ, Comstock GW, Morris JS (1989) Cancer Res 49:6144–6148
Ozmutlu S, Dede S, Ceylan E (2012) JRAAS 13:328–333
Veeramachaneni S, Ausman LM, Choi SW, Russell RM, Wang X-D (2008) J Nutr 138:1329–1335
Yaping Z, Suping Q, Wenli Y, Zheng X, Hong S, Side Y, Dapu W (2002) Food Chem 77:209–212
Stahl W, Sundquist AR, Hanusch M, Schwarz W, Sies H (1993) Clin Chem 39:810–814
Chasse GA, Mak ML, Deretey E, Farkas I, Torday LL, Papp JG, Sharma DSR, Agarwal A, Chakravarthi S, Agarwal S, Rao AV (2001) J Mol Str (Theochem) 571:27–37
Chasse GA, Chasse KP, Kucsman A, Torday LL, Papp JG (2001) J Mol Str(Theochem) 571:7–26
Guo J-J, Hsieh H-Y, Hu C-H (2009) J Phys Chem B 113:15699–15708
Bruskov VI, Malakhova LV, Masalimov ZK, Chernikov AV (2002) Nucl Acid Res 30:1354–1363
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117–129
Cossi M, Scalmani G, Rega N, Barone V (2002) J Chem Phys 117:43–54
Ess DH, Houk KN (2005) J Phys Chem A 109:9542–9553
Silva PJ, Ramos MJ (2011) Comput Theor Chem 966:120–126
Sousa SF, Fernandes PA, Ramos MJ (2007) J Phys Chem A 111:10439–10452
Wodrich MD, Corminboeuf C, Schleyer PR (2006) Org Lett 8:3631–3634
Perdrew JP, Ziesche Ed P, Eschrig H (1991) Akad Verlag 91:11–20
Levine IN (1994) Quantum chemistry, 4th edn. Prentice-Hall, Englewood Cliffs, NJ
Ramalho SS, Vilela AFA, Barreto PRP, Gargano R (2005) Chem Phys Lett 413:151–156
Carstensen HH, Dean AM (2009) J Phys Chem A 113:367–380
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Hratchian X, Li HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski R, Martin RL, Morokuma K, Zakrzewski VG, Voth G A, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz J V, Cioslowski J, Fox DJ (2009), Gaussian 09, revisionA.1. Gaussian Inc, Wallingford, CT
Frisch AE, Dennington RD, Keith TA, Nielsen AB, Holder AJ (2003) Gauss View, rev. 3.9. Gaussian Inc, Pittsburg, PA
Boileau TWM, Boileau AC, Erdman JW Jr (2002) Exp Biol Med 227:914–919
Acknowledgments
The authors are thankful to the University Grants Commission (New Delhi) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 42 kb)
Rights and permissions
About this article
Cite this article
Prasad, A.K., Mishra, P.C. Modeling the mechanism of action of lycopene as a hydroxyl radical scavenger. J Mol Model 20, 2233 (2014). https://doi.org/10.1007/s00894-014-2233-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2233-5