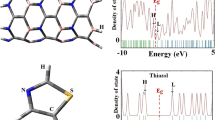
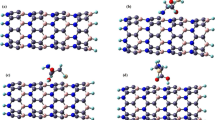
Abstract
Using density functional theory calculations, we investigated properties of a functionalized BC2N nanotube with NH3 and five other NH2-X molecules in which one of the hydrogen atoms of NH3 is substituted by X = −CH3, −CH2CH3, −COOH, −CH2COOH and −CH2CN functional groups. It was found that NH3 can be preferentially adsorbed on top of the boron atom, with adsorption energy of −12.0 kcal mol−1. The trend of adsorption-energy change can be correlated with the trend of relative electron-withdrawing or -donating capability of the functional groups. The adsorption energies are calculated to be in the range of −1.8 to −14.2 kcal mol−1, and their relative magnitude order is found as follows: H2N(CH2CH3) > H2N(CH3) > NH3 > H2N(CH2COOH) > H2N(CH2CN) > H2N(COOH). Overall, the functionalization of BC2N nanotube with the amino groups results in little change in its electronic properties. The preservation of electronic properties of BC2N coupled with the enhancement of solubility renders their chemical modification with either NH3 or amino functional groups to be a way for the purification of BC2N nanotubes.




Similar content being viewed by others
References
Iijima S (1991) Nature 354:56–58
Sun SL, Hu YY, Xu HL, Su ZM, Hao LZ (2012) J Mol Model 18:3219–3225
Politzer P, Murray JS, Lane P, Concha MC, Jin P, Peralta-Inga Z (2005) J Mol Model 11:258–264
Chełmecka E, Pasterny K, Kupka T, Stobiński L (2012) J Mol Model 18:2241–2246
Redlich P, Loeffler J, Ajayan PM, Bill J, Aldinger F, Ruhle M (1996) Chem Phys Lett 260:465–470
Zhang Y, Gu H, Suenaga K, Iijima S (1997) Chem Phys Lett 279:264–269
Zhang Y, Suenaga K, Colliex C, Iijima S (1998) Science 281:973–975
Rossato J, Baierle RJ (2007) Phys Rev B 75:235401–235407
Pan H, Feng YP, Lin JY (2006) Phys Rev B 73:035420–035425
Hernndez E, Goze C, Bernier P, Rubio A (1998) Phys Rev Lett 80:4502–4505
Sen R, Satishkumar BC, Govindaraj A, Harikumar KR, Gargi R, Zhang JP, Cheetham AK, Rao CNR (1998) Chem Phys Lett 287:671–676
Redlich P, Loeffler J, Ajayan PM, Bill J, Aldinger F, Rühle M (1996) Chem Phys Lett 26:465–470
Bai XD, Guo JD, Yu J, Wang EG, Yuan J, Zhou WZ (2000) Appl Phys Lett 76:2624–2626
Raidongia K, Jagadeesan D, Upadhyay-Kahaly M, Waghmare UV, Pati SK, Eswaramoorthy M, Rao CNR (2008) J Mater Chem 18:83–90
Niyogi S, Hamon MA, Hu H, Zhao B, Bhowmik P, Sen R, Itkis ME, Haddon RC (2002) Accounts Chem Res 35:1105–1113
Ahmadi Peyghan A, Omidvar A, Hadipour NL, Bagheri Z, Kamfiroozi M (2012) Physica E 44:1357–1360
Beheshtian J, Peyghan AA, Bagheri Z (2012) Sens Actuators B: Chem 171-172:846–852
Beheshtian J, Baei MT, Peyghan AA, Bagheri Z (2012) J Mol Model 18:4745–4750
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2012) Struct Chem 23:653–657
Beheshtian J, Soleymanabadi H, Kamfiroozi M, Ahmadi A (2012) J Mol Model 18:2343–2348
Beheshtian J, Peyghan AA, Bagheri Z (2012) Physica E 44:1963–1968
Zhou Z, Zhao J, Gao X, Chen Z, Yan J, Schleyer PR, Morinaga M (2005) Chem Mater 17:992–1000
Schmidt M et al (1993) J Comput Chem 14:1347–1363
Moradi M, Peyghan A, Bagheri Z, Kamfiroozi M (2012) J Mol Model 18:3535–3540
Beheshtian J, Peyghan A, Bagheri Z, Kamfiroozi M (2012) Struct Chem 5:1567–1572
Beheshtian J, Peyghan AA, Bagheri Z (2012) Comput Theor Chem 992:164–167
Wanbayor R, Ruangpornvisuti V (2012) Appl Surf Sci 258:3298–3301
Beheshtian J, Peyghan AA, Bagheri Z (2012) Appl Surf Sci 258:8171–8176
Tomić S, Montanari B, Harrison NM (2008) Physica E 40:2125–2127
O’Boyle N, Tenderholt A, Langner K (2008) J Comput Chem 29:839–845
Olmsted J, Williams GM (1997) Chemistry: the molecular science. WCB, Iowa
Zhang J, Wang X, Yang W, Yu W, Feng T, Li Q, Liu X, Yang C (2006) Carbon 44:418–422
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beheshtian, J., Ahmadi Peyghan, A. Theoretical study on the functionalization of BC2N nanotube with amino groups. J Mol Model 19, 2211–2216 (2013). https://doi.org/10.1007/s00894-013-1759-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1759-2