Abstract
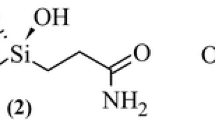
DFT (B3LYP and M06L) as well as ab initio (MP2) methods with Dunning cc-pVnZ (n = 2,3) basis sets are employed for the study of the binding ability of the new class of protease inhibitors, i.e., silanediols, in comparison to the well-known and well-studied class of inhibitors with hydroxamic functionality (HAM). Active sites of metalloproteases are modeled by [R3M-OH2]2+ complexes, where R stands for ammonia or imidazole molecules and M is a divalent cation, namely zinc, iron or nickel (in their different spin states). The inhibiting activity is estimated by calculating Gibbs free energies of the water displacement by metal binding groups (MBGs) according to: [R3M-OH2]2+ + MBG → [R3M-MBG]2+ + H2O. The binding energy of silanediol is only a few kcal mol−1 inferior to that of HAM for zinc and iron complexes and is even slightly higher for the triplet state of the (NH3)3Ni2+ complex. For both MBGs studied in the ammonia model the binding ability is nearly the same, i.e., Fe2+(t) > Ni2+(t) > Fe2+(q) > Ni2+(s) > Zn2+. However, for the imidazole model the order is slightly different, i.e., Ni2+(t) > Fe2+(t) > Fe2+(q) > Ni2+(s) ≥ Zn2+. Equilibrium structures of the R3Zn 2+ complexes with both HAM and silanediol are characterized by the monodentate binding, but the bidentate character of binding increases on going to iron and nickel complexes. Two types of intermediates of the water displacement reactions for [(NH3)3M-OH2]2+ complexes were found which differ by the direction of the attack of the MBG. Hexacoordinated complexes exhibit bidentate bonding of MBGs and are lower in energy for M=Ni and Fe. For Zn penta- and hexacoordinated complexes have nearly the same energy. Intermediate complexes with imidazole ligands have only octahedral structures with bidentate bonding of both HAM and dimethylsilanediol molecules.














Similar content being viewed by others
References
Vallee BL, Auld DS (1993) Zinc: biological function and coordination motifs. Acc Chem Res 26:543–551
Maret W, Li Y (2009) Coordination dynamics of zinc in proteins. Chem Rev 109:4682–4707
Lipscomb WN, Sträter N (1996) Recent advances in zinc enzymology. Chem Rev 96:2375–2433
Holm RH, Kennepohl P, Solomon EI (1996) Structural and functional aspects of metal sites in biology. Chem Rev 96:2239–2314
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401:188–193
Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, Paolini C, De Francesco R, Gallinari P, Steinkuhler C, Di Marco S (2004) Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc Natl Acad Sci USA 101:15064–15069
Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, Jennings AJ, Luong C, Arvai A, Buggy JJ, Chi E, Tang J, Sang BC, Verner E, Wynands R, Leahy EM, Dougan DR, Snell G, Navre M, Knuth MW, Swanson RV, McRee DE, Taric LW (2004) Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12:1325–1334
Becker A, Schlichting I, Kabsch W, Groche D, Schultz S, Wagner AFV (1998) Iron center, substrate recognition, and mechanism of peptide deformylase. Nat Struct Biol 5:1053–1058
Madison V, Duca J, Bennett F, Bohanon S, Cooper A, Chu M, Desai J, Girijavallabhan V, Hare R, Hruza A, Hendrata S, Huang Y, Kravec C, Malcolm B, McCormick J, Miesel L, Ramamanathan L, Reichert P, Saksena A, Wang J, Weber PC, Zhu H, Fischmann T (2002) Binding affinities and geometries of various metal ligands in peptide deformylase inhibitors. Biophys Chem 101–102:239–247
Natesh R, Schwager SLU, Sturrock ED, Acharya KR (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421:551–554
Christianson DW, Lipscomb WN (1989) Carboxypeptidase A. Acc Chem Res 22:62–69
Mock WL, Zhang JZ (1991) Mechanistically significant diastereoselection in the sulfoximine inhibition of carboxypeptidase A. J Biol Chem 266:6393–6400
Fersht AR (1999) Enzyme structure and mechanism in protein science. Freeman, New York
Wu S, Zhang C, Xu D, Guo H (2010) Catalysis of carboxypeptidase A: promoted-water vs nucleophilic pathways. J Phys Chem B114:9259–9267
Gupta SP (2007) Quantitative structure-activity relationship studies on zinc-containing metalloproteinase inhibitors. Chem Rev 107:3042–3087
Farkas E, Katz Y, Bhusare S, Reich R, Röschenthaler G-V, Königsmann M, Breuer E (2004) Carbamoylphosphonate Based Matrix Metalloproteinase (MMP) inhibitor metal complexes - solution studies and stability constants. Towards a zinc selective binding group. J Biol Inorg Chem 9:307–315
Massie BM (1998) 15 years of heart-failure trials: what have we learned. Lancet 352(suppl I):SI29–33
Burnier M, Brunner HR (2000) Angiotensin II receptor antagonists. Lancet 355:637–45
Babine RE, Bender SL (1997) Molecular recognition of proteinminus signligand complexes: applications to drug design. Chem Rev 97:1359–1472
Vane JR (1999) The history of inhibitors of angiotensin converting enzyme. J Physiol Pharmacol 50:489–498
Borkakoti N (2004) Matrix metalloprotease inhibitors: design from structure. Biochem Soc Trans 32:17–22
Sieburth SM, Nittoli T, Mutahi AM, Guo L (1998) Silanediols-A new class of potent protease inhibitor. Angew Chem Int Ed 37:812–814
Chen CA, Sieburth SM, Glekas A, Hewitt GW, Trainor GL, Erickson-Viitanen S, Garber SS, Cordova B, Jeffry S, Klabe RM (2001) Drug design with a new transition state analog of the hydrated carbonyl: silicon-based inhibitors of the HIV protease. Chem Biol 8:1161–1166
Mutahi MW, Nittoli T, Guo L, Sieburth SM (2002) Silicon-based metalloprotease inhibitors: synthesis and evaluation of silanol and silanediol peptide analogues as inhibitors of angiotensin-converting enzyme. J Am Chem Soc 124:7363–7375
Kim J, Glekas A, Sieburth SM (2002) Silanediol-based inhibitor of thermolysin. Bioorg Med Chem Lett 12:3625
Juers DH, Kim J, Matthews BW, Sieburth SM (2005) Structural analysis of silanediols as transition-state-analogue inhibitors of the benchmark metalloprotease thermolysin. Biochem 44:16524–16528
El Yazal J, Pang YP (1999) Novel stable configurations and tautomers of the neutral and deprotonated hydroxamic acids predicted from high-level Ab initio calculations. J Phys Chem A 103:8346–8350
El Yazal J, Pang YP (1999) Ab initio calculations of proton dissociation energies of zinc ligands: hypothesis of imidazolate as zinc ligand in proteins. J Phys Chem B 103:8773–8779
El Yazal J, Pang YP (2000) Proton dissociation energies of zinc-coordinated hydroxamic acids and their relative affinities for zinc: insight into design of inhibitors of zinc- containing proteinases. J Phys Chem B 104:6499–6504
Deerfield DW, Carter C, Pedersen LG (2001) Models for protein–zinc ion binding sites II. The catalytic sites. Int J Quant Chem 83:150–165
Remko M, Garaj V (2003) Thermodynamics of binding of Zn2+ to carbonic anhydrase inhibitors. Mol Phys 101:2357–2368
Cheng F, Zhang R, Luo X, Shen J, Li X, Gu J, Zhu W, Shen J, Sagi I, Ji R, Chen K, Jiang H (2002) Quantum chemistry study on the interaction of the exogenous ligands and the catalytic zinc ion in matrix metalloproteinases. J Phys Chem B 106:4552–4559
Khandelwal A, Lukacova V, Comez D, Kroll DM, Raha S, Balaz S (2005) A combination of docking, QM/MM, and MD simulation for binding affinity estimation of metalloprotein ligands. J Med Chem 48:5437–5447
Linder DP, Rodgers KR (2004) Theoretical study of imidazole- and thiol-based zinc binding groups relevant to inhibition of metzincins. J Phys Chem B 108:13839–13849
Šramko M, Garaj V, Remko M (2008) Thermodynamics of binding of angiotensin-converting enzyme inhibitors to enzyme active site model. J Mol Struct (THEOCHEM) 869:19–28
Dobbs KD, Rinehart AM, Howard MH, Zheng YJ, Kleier DA (2006) Computational characterization of metal binding groups for metalloenzyme inhibitors. J Chem Theor Comput 2:990–996
Vanommeslaeghe K, Loverix S, Geerlings P, Tourwé D (2005) DFT-based ranking of zinc-binding groups in histone deacetylase inhibitors. Bioorg Med Chem 13:6070–6082
Vanommeslaeghe K, Van Alsenoy C, De Proft F, Martins JC, Tourwé D, Geerlings P (2003) Ab initio study of the binding of Trichostatin A (TSA) in the active site of histone deacetylase like protein (HDLP). Org Biomol Chem 1:2951–2957
Vanommeslaeghe K, De Proft F, Loverix S, Tourwé D, Geerlings P (2005) Theoretical study revealing the functioning of a novel combination of catalytic motifs in histone deacetylase. Bioorg Med Chem 13:3987–3992
Corminboef C, Hu P, Tuckerman ME, Zhang Y (2006) Unexpected deacetylation mechanism suggested by a density functional theory QM/MM study of histone-deacetylase-like protein. J Am Chem Soc 128:4530–4531
Frison G, Ohanessian G (2008) A comparative study of semiempirical, ab initio, and DFT methods in evaluating metal-ligand bond strength, proton affinity, and interactions between first and second shell ligands in Zn-biomimetic complexes. J Comput Chem 29:416–433
Elstner M, Cui Q, Munih P, Kaxiras E, Frauenheim T, Karplus M (2003) Modeling Zinc in biomolecules with the self consistent charge-density functional tight binding (SCC-DFTB) method: Applications to structural and energetic analysis. J Comput Chem 24:565–581
Marmion CJ, Griffith D, Nolan KB (2004) Hydroxamic acids - an intriguing family of bioligands and enzyme inhibitors. Eur J Inorg Chem 15:3003–3016
Codd R (2008) Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord Chem Rev 252:1387–1408
Wang D, Helquist P, Wiest O (2007) Zinc binding in HDAC inhibitors: a DFT study. J Org Chem 72:5446–5449
Wu R, Lu Z, Cao Z, Zhang Y (2011) Zinc chelation with hydroxamate in histone deacetylases modulated by water access to the linker binding channel. J Am Chem Soc 133:6110–6113
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys Rev B 37:785–789
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Amin EA, Truhlar DG (2008) Zn coordination chemistry: development of benchmark suites for geometries, dipole moments, and bond dissociation energies and their use to test and validate density functionals and molecular orbital theory. J Chem Theor Comput 4:75–85
Sorkin A, Iron MA, Truhlar DG (2008) Density functional theory in transition-metal chemistry: relative energies of low-lying states of iron compounds and the effect of spatial symmetry breaking. J Chem Theor Comput 4:307–315
Cramer CJ, Truhlar DG (2009) Density functional theory for transition metals and transition metal chemistry. Phys Chem Chem Phys 11:10757–10816
Zeng Y, Wang S, Feng H, Xie Y, King RB (2011) Highly unsaturated binuclear butadiene iron carbonyls: quintet spin States, perpendicular structures, agostic hydrogen atoms, and iron-iron multiple bonds. Int J Mol Sci 12:2216–2231
Binkley JS, Pople JA (1975) Møller–Plesset theory for atomic ground state energies. Int J Quant Chem 9:229–236
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Woon DE, Dunning TH (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358–1371
Frisch MJ et al. (2009) Gaussian 09, Revision B.01. Gaussian, Wallingford
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1988) NBO v3.1, Madison
Akesson R, Pettersson LGM, Sandstrom M, Siegbahn PEM, Wahlgrent U (1993) Theoretical study of water-exchange reactions for the divalent ions of the first transition period. J Phys Chem 97:3765–3774
Dudev T, Lim C (2003) Principles governing Mg, Ca, and Zn binding and selectivity in proteins. Chem Rev 103:773–788
He H, Puerta DT, Cohen SM, Rodgers KR (2005) Spectroscopic study of reactions between chelating zinc-binding groups and mimics of the MMP and ADAM catalytic sites: the coordination chemistry of metalloprotease inhibition. Inorg Chem 44:7431–7442
Bertrand P (2010) Inside HDAC with HDAC inhibitors. Eur J Med Chem 45:2095–2116
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ignatyev, I.S., Montejo, M., Rodríguez Ortega, P.G. et al. Quantum chemical study of silanediols as metal binding groups for metalloprotease inhibitors. J Mol Model 19, 1819–1834 (2013). https://doi.org/10.1007/s00894-012-1745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1745-0