Abstract
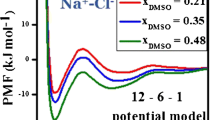
Ion clustering and the solvation properties in the NaCl solutions are explored by molecular dynamics simulations with several popular force fields. The existence of ions has a negligible disturbance to the hydrogen bond structures and rotational mobility of water beyond the first ion solvation shells, which is suggested by the local hydrogen bond structures and the rotation times of water. The potential of mean force (PMF) of ion pair in the dilute solution presents a consistent view with the populations of ion clusters in the electrolyte solutions. The aggregation level of ions is sensitive to the force field used in the simulations. The ion-ion interaction potential plays an important role in the forming of the contact ion pair. The entropy of water increases as the ion pair approaches each other and the association of ion pair is driven by the increment of water entropy according to the results from the selected force fields. The kinetic transition from the single solvent separated state to the contact ion pair is controlled by the enthalpy loss of solution.

Ion pairing and ion induction to solvent play an important role in the protein folding and chemical reactions in the water solutions. The existence of ions has a negligible disturbance to the hydrogen bond structures and rotational mobility of water beyond the first ion solvation shells in the NaCl solutions. The clustering level of ions is sensitive to the force field used in the simulations. The formation of NaCl ion pair in the dilute solution is driven by the entropy increment of water








Similar content being viewed by others
References
Marcus Y, Hefter G (2006) Ion pairing. Chem Rev 106:4585–4621
Fuoss RM (1980) PNAS 77:34–38
Omta AW, Kropman MF, Woutersen S, Bakker HJ (2003) Science 301:347–349
Skinner JL (2010) Science 328:985–986
Tielrooij KJ, Garcia-Araez N, Bonn M, Bakker HJ (2010) Science 328:1006–1009
Chandra A (2000) Phys Rev Lett 85:768–771
Carrillo-Tripp M, Saint-Martin H, Ortega-Blake I (2003) J Chem Phys 118:7062–7073
Mancinelli R, Botti A, Bruni F, Ricci MA, Soper AK (2007) Phys Chem Chem Phys 9:2959–2967
Collins KD, Neilson GW, Enderby JE (2007) Biophys Chem 128:95–104
Smith DE, Dang LX (1994) J Chem Phys 100:3757–3766
Degrève L, da Silva FLB (1999) J Chem Phys 111:5150–5156
Chen AA, Pappu RV (2007) J Phys Chem B 111:6469–6478
Gu B, Zhang FS, Wang ZP, Zhou HY (2008) J Chem Phys 129:184505–184507
Fennell CJ, Bizjak A, Vlachy V, Dill KA (2009) J Phys Chem B 113:6782–6791
Timko J, Bucher D, Kuyucak S (2010) J Chem Phys 132:114510
Auffinger P, Cheatham TE III, Vaiana AC (2007) J Chem Theory Comput 3:1851–1859
Adams DJ, McDonald IR (1974) J Phys C 7:2761–2775
Huggins ML, Mayer JE (1933) J Chem Phys 1:643–646
Tosi M, Fumi F (1964) J Phys Chem Solids 25:45–52
Åqvist J (1990) J Phys Chem 94:8021–8024
Beglov D, Roux B (1994) J Chem Phys 100:9050–9063
Jensen KP, Jorgensen WL (2006) J Chem Theor Comput 2:1499–1509
Straatsma TP, Berendsen HJC (1988) J Chem Phys 89:5876–5886
Berendsen HJC, Grigera JR, Straatsma TP (1987) J Phys Chem 91:6269–6271
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79:926–935
Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J (1981) In: Pullman B (ed) Intermolecular forces. Reidel, Dordrecht, p 331
Sanza E, Vega C (2007) J Chem Phys 126:014507
Anwar J, Frenkel D, Noro MG (2003) J Chem Phys 118:728–735
Berendsen HJC, Postma JPM, van Gunsteren WF, Di Nola A, Hauk JR (1984) J Chem Phys 81:3684–3690
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) J Chem Phys 103:8577–8593
Ponder JW, Richards FM (1987) J Comput Chem 8:1016–1024
Hess B, Holm C, van der Vegt N (2006) J Chem Phys 124:164509
Li J, Car R, Tang C, Wingreen NS (2007) PNAS 104:2626–2630
Varma S, Rempe SB (2006) Biophys Chem 124:192–199
Chowdhuri S, Chandra A (2003) J Chem Phys 118:9719–9725
Laage D, Hynes JT (2006) Science 311:832–835
Mancinelli R, Botti A, Bruni F, Ricci MA, Soper AK (2007) J Phys Chem B 111:13570–13577
Yang L, Fan Y, Gao YQ (2011) J Phys Chem B 115:12456–12465
Mark P, Nilsson L (2001) J Phys Chem A 105:9954–9960
Chialvo AA, Simonson JM (2003) J Chem Phys 118:7921–7929
Hassan SA (2008) J Phys Chem B 112:10573–10584
Horinek D, Mamatkulov SI, Netz RR (2009) J Chem Phys 130:124507–124521
Fyta M, Netz RR (2012) J Chem Phys 136:124103
Acknowledgments
The authors greatly thank Professor Jay William Ponder for providing the Tinker program. This work is supported by National Natural Science Foundation of China (No. 20873055, 21176029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, X. & Zhao, DX. Ion disturbance and clustering in the NaCl water solutions. J Mol Model 19, 661–672 (2013). https://doi.org/10.1007/s00894-012-1581-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1581-2