Abstract
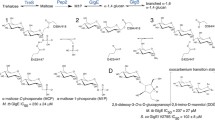
Enzyme catalyzed phosphate transfer is a part of almost all metabolic processes. Such reactions are of central importance for the energy balance in all organisms and play important roles in cellular control at all levels. Mutases transfer a phosphoryl group while nucleases cleave the phosphodiester linkages between two nucleotides. The subject of our present study is the Lactococcus lactis β-phosphoglucomutase (β-PGM), which effectively catalyzes the interconversion of β-D-glucose-1-phosphate (β-G1P) to β-D-glucose-6-phosphate (β-G6P) and vice versa via stabile intermediate β-D-glucose-1,6-(bis)phosphate (β-G1,6diP) in the presence of Mg2+. In this paper we revisited the reaction mechanism of the phosphoryl transfer starting from the bisphosphate β-G1,6diP in both directions (toward β-G1P and β-G6P) combining docking techniques and QM/MM theoretical method at the DFT/PBE0 level of theory. In addition we performed NEB (nudged elastic band) and free energy calculations to optimize the path and to identify the transition states and the energies involved in the catalytic cycle. Our calculations reveal that both steps proceed via dissociative pentacoordinated phosphorane, which is not a stabile intermediate but rather a transition state. In addition to the Mg2+ ion, Ser114 and Lys145 also play important roles in stabilizing the large negative charge on the phosphate through strong coordination with the phosphate oxygens and guiding the phosphate group throughout the catalytic process. The calculated energy barrier of the reaction for the β-G1P to β-G1,6diP step is only slightly higher than for the β-G1,6diP to β-G6P step (16.10 kcal mol-1 versus 15.10 kcal mol-1) and is in excellent agreement with experimental findings (14.65 kcal mol-1).

Enzymatic phosphoryl transfer of β-phosphoglucomutase







Similar content being viewed by others
References
Cleland WW, Hengge AC (2006) Enzymatic mechanisms of phosphate and sulfate transfer. Chem Rev 106:3252–3278
Holmes RR (2004) Phosphoryl transfer enzymes and hypervalent phosphorus chemistry. Acc Chem Res 37:746–753
Knowles JR (1980) Enzyme-catalyzed phosphoryl transfer-reactions. Annu Rev Biochem 49:877–919
Ma C, Ray WJ (1980) Structural comparisons among the central complexes in the phosphoglucomutase system by means of spectral techniques. Biochem 19:751–759
Ray WJ, Long JW, Owens JD (1976) Analysis of substrate-induced rate effect in phosphoglucomutase system. Biochem 15:4006–4017
Zhang GF, Dai J, Wang LB, Dunaway-Mariano D, Tremblay LW, Allen KN (2005) Catalytic cycling in beta-phosphoglucomutase: a kinetic and structural analysis. Biochem 44:9404–9416
Dai JY, Finci L, Zhang CC, Lahiri S, Zhang GF, Peisach E, Allen KN, Dunaway-Mariano D (2009) Analysis of the structural determinants underlying discrimination between substrate and solvent in beta-phosphoglucomutase catalysis. Biochem 48:1984–1995
Dai JY, Wang LB, Allen KN, Radstrom P, Dunaway-Mariano D (2006) Conformational cycling in beta-phosphoglucomutase catalysis: reorientation of the beta-d-glucose 1,6-(bis) phosphate intermediate. Biochem 45:7818–7824
Lahiri SD, Zhang GF, Dunaway-Mariano D, Allen KN (2003) The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction. Science 299:2067–2071
Baxter NJ, Hounslow AM, Bowler MW, Williams NH, Blackburn GM, Waltho JP (2009) Mgf3- and alpha-galactose 1-phosphate in the active site of beta-phosphoglucomutase form a transition state analogue of phosphoryl transfer. J Am Chem Soc 131:16334–16335
Golicnik M, Olguin LF, Feng GQ, Baxter NJ, Waltho JP, Williams NH, Hollfelder F (2009) Kinetic analysis of beta-phosphoglucomutase and its inhibition by magnesium fluoride. J Am Chem Soc 131:1575–1588
Tremblay LW, Zhang GF, Dai JY, Dunaway-Mariano D, Allen KN (2005) Chemical confirmation of a pentavalent phosphorane in complex with beta-phosphoglucomutase. J Am Chem Soc 127:5298–5299
Webster C (2004) High-energy intermediate or stable transition state analogue: Theoretical perspective of the active site and mechanism of beta-phosphoglucomutase. J Am Chem Soc 126:6840–6841
Marcos E, Field MJ, Crehuet R (2010) Pentacoordinated phosphorus revisited by high-level qm/mm calculations. Proteins 78:2405–2411
Valiev M, Garrett BC, Tsai MK, Kowalski K, Kathmann SM, Schenter GK, Dupuis M (2007) Hybrid approach for free energy calculations with high-level methods: Application to the s(n)2 reaction of chcl3 and oh- in water. J Chem Phys 127:51102
Valiev M, Kawai R, Adams JA, Weare JH (2003) The role of the putative catalytic base in the phosphoryl transfer reaction in a protein kinase: first-principles calculations. J Am Chem Soc 125:9926–9927
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, Van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL, de Jong WA (2010) Nwchem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181:1477–1489
Kamerlin SCL, Florian J, Warshel A (2008) Associative versus dissociative mechanisms of phosphate monoester hydrolysis: on the interpretation of activation entropies. Chemphyschem 9:1767–1773
Allen KN, Dunaway-Mariano D, Lahiri SD, Tremblay L, Zhang GF, Dai JY (2004) Mechanism of phosphoryl transfer in a phosphomutase. Abstr Pap Am Chem S 228:U304–U305
Kamerlin SCL, Wilkie J (2007) The role of metal ions in phosphate ester hydrolysis. Org Biomol Chem 5:2098–2108
Henkelman G, Jonsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys 113:9978–9985
Henkelman G, Uberuaga BP, Jonsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113:9901–9904
Moe 2010.10 (http://www.chemcomp.com)
Gordon JC, Myers JB, Folta T, Shoja V, Heath LS, Onufriev A (2005) H++: A server for estimating pk(a)s and adding missing hydrogens to macromolecules. Nucleic Acids Res 33:W368–W371
Elsaesser B, Valiev M, Weare JH (2009) A dianionic phosphorane intermediate and transition states in an associative a(n) + d-n mechanism for the ribonucleasea hydrolysis reaction. J Am Chem Soc 131:3869–3871
Elsaesser B, Fels G (2010) Atomistic details of the associative phosphodiester cleavage in human ribonuclease h. Phys Chem Chem Phys 12:11081–11088
Valiev M, Yang J, Adams JA, Taylor SS, Weare JH (2007) Phosphorylation reaction in capk protein kinase-free energy quantum mechanical/molecular mechanics simulations. J Phys Chem B 111:13455–13464
Kroemer RT, Vulpetti A, McDonald JJ, Rohrer DC, Trosset JY, Giordanetto F, Cotesta S, McMartin C, Kihlen M, Stouten PFW (2004) Assessment of docking poses: interactions-based accuracy classification (ibac) versus crystal structure deviations. J Chem Inf Comput Sci 44:871–881
Suhre K, Sanejouand YH (2004) Elnemo: A normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids Res 32:W610–W614
Lindahl E, Azuara C, Koehl P, Delarue M (2006) Nomad-ref: visualization, deformation and refinement of macromolecular structures based on all-atom normal mode analysis. Nucleic Acids Res 34:W52–W56
Bowler MW, Cliff MJ, Waltho JP, Blackburn GM (2010) Why did nature select phosphate for its dominant roles in biology? New J Chem 34:784–794
Baxter NJ, Bowler MW, Alizadeh T, Cliff MJ, Hounslow AM, Wu B, Berkowitz DB, Williams NH, Blackburn GM, Waltho JP (2010) Atomic details of near-transition state conformers for enzyme phosphoryl transfer revealed by mgf(3)(-) rather than by phosphoranes. Proc Natl Acad Sci USA 107:4555–4560
Tama F, Sanejouand YH (2001) Conformational change of proteins arising from normal mode calculations. Protein Eng 14:1–6
Berente I, Beke T, Naray-Szabo G (2007) Quantum mechanical studies on the existence of a trigonal bipyramidal phosphorane intermediate in enzymatic phosphate ester hydrolysis. Theor Chem Acc 118:129–134
Acknowledgments
The calculations were carried out at the Paderborn Center for Parallel Computing (PC2) and at the Molecular Science Computing Facility in the Environmental Molecular Sciences Laboratory User Facility sponsored by the United Stated Department of Energy. The authors acknowledge Marat Valiev for the normal mode calculations on the transition state structures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Structures of NEB beads and stationary points is available free of charge

ESM 1
(JPEG 325 kb)

ESM 2
(JPEG 322 kb)

ESM 3
(JPEG 326 kb)

ESM 4
(JPEG 328 kb)

ESM 5
(JPEG 328 kb)

ESM 6
(JPEG 326 kb)

ESM 7
(JPEG 331 kb)

ESM 8
(JPEG 323 kb)

ESM 9
(JPEG 321 kb)

ESM 10
(JPEG 325 kb)

ESM 11
(JPEG 325 kb)

ESM 12
(JPEG 323 kb)

ESM 13
(JPEG 322 kb)

ESM 14
(JPEG 322 kb)

ESM 15
(JPEG 325 kb)

ESM 16
(JPEG 324 kb)

ESM 17
(JPEG 324 kb)

ESM 18
(JPEG 322 kb)

ESM 19
(JPEG 322 kb)

ESM 20
(JPEG 319 kb)

ESM 21
(JPEG 319 kb)

ESM 22
(JPEG 318 kb)

ESM 23
(JPEG 318 kb)

ESM 24
(JPEG 317 kb)

ESM 25
(JPEG 318 kb)

ESM 26
(JPEG 318 kb)

ESM 27
(JPEG 318 kb)

ESM 28
(JPEG 322 kb)

ESM 29
(JPEG 323 kb)

ESM 30
(JPEG 322 kb)
Rights and permissions
About this article
Cite this article
Elsässer, B., Dohmeier-Fischer, S. & Fels, G. Theoretical investigation of the enzymatic phosphoryl transfer of β-phosphoglucomutase: revisiting both steps of the catalytic cycle. J Mol Model 18, 3169–3179 (2012). https://doi.org/10.1007/s00894-011-1344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1344-5