Abstract
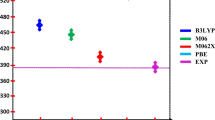
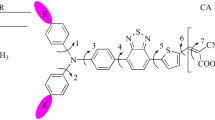
The molecular structures and absorption spectra of triphenylamine dyes containing different numbers of anchoring groups (S1-S3) were investigated by density functional theory (DFT) and time-dependent DFT. The calculated geometries indicate that strong conjugation is formed in the dyes. The interfacial charge transfer between the TiO2 electrode and S1-S3 are electron injection processes from the excited dyes to the semiconductor conduction band. The simulated absorption bands are assigned to π → π* transitions according to the qualitative agreement between the experimental and calculated results. The effect of anchoring group number on the molecular structures, absorption spectra and photovoltaic performance were comparatively discussed.






Similar content being viewed by others
References
O'Regan B, Grätzel M (1991) Nature 353:737–740
Nazeeruddin MK, Kay A, Rodicio, Humpbry-Baker R, Miiller E, Liska P, Vlachopoulos N, Grätzel M (1993) J Am Chem Soc 115:6382–6390
Nazeeruddin MK, Péchy P, Renouard T, Zakeeruddin SM, Humphry-Baker R, Comte P, Liska P, Cevey L, Costa E, Shklover V, Spiccia L, Deacon GB, Bignozzi CA, Grätzel M (2001) J Am Chem Soc 123:1613–1624
Nazeeruddin MK, Angelis FD, Fantacci S, Selloni A, Viscardi G, Liska P, Ito S, Takeru B, Grätzel M (2005) J Am Chem Soc 127:16835–16847
Gao F, Wang Y, Shi D, Zhang J, Wang M, Jing X, Humphry-Baker R, Wang P, Zakeeruddin SM, Grätzel M (2008) J Am Chem Soc 130:10720–10728
Chen CY, Wang M, Li JY, Pootrakulchote N, Alibabaei L, Ngoc-le CH, Decoppet JD, Tsai JH, Grätzel C, Wu CG, Zakeeruddin SM, Grätzel M (2009) ACS Nano 3:3103–3109
Amao Y, Komori T (2004) Biosensors Bioelectron 19:843–847
Horiuchi T, Miura H, Sumioka K, Uchida S (2004) J Am Chem Soc 126:12218–12219
Hara K, Kurashige M, Dan-oh Y, Kasada C, Shinpo A, Suga S, Sayama K, Arakawa H (2003) New J Chem 27:783–785
Horiuchi T, Miura H, Uchida S (2003) Chem Commun 3036–3037
Horiuchi T, Miura H, Uchida S (2004) J Photochem Photobiol A Chem 164:29–32
Kitamura T, Ikeda M, Shigaki K, Inoue T, Anderson NA, Ai X, Lian T, Yanagida S (2004) Chem Mater 16:1806–1812
Hagberg DP, Yum J-H, Lee H, Angelis FD, Marinado T, Karlsson KM, Humphry-Baker R, Sun L, Hagfeldt A, Grätzel M, Nazeeruddin MK (2008) J Am Chem Soc 130:6259–6266
Tian H, Yang X, Chen R, Zhang R, Hagfeldt A, Sun L (2008) J Phys Chem C 112:11023–11033
Mishra A, Fischer MKR, Bäuerle P (2009) Angew Chem Int Edn 48:2474–2499
Ning Z, Tian H (2009) Chem Commun 5483–5495
Shang H, Luo Y, Guo X, Huang X, Zhan X, Jiang K, Meng Q (2010) Dyes Pigm 87:249–256
Barolo C, Nazeeruddin MK, Fantacci S, Di Censo D, Comte P, Liska P, Viscardi G, Quagliotto P, De Angelis F, Ito S, Gratzel M (2006) Inorg Chem 45:4642–4653
Onozawa-Komatsuzaki N, Kitao O, Yanagida M, Himeda Y, Sugihara H, Kasuga K (2006) New J Chem 30:689–697
Monat JE, Rodriguez JH, McCusker JK (2002) J Phys Chem A 106:7399–7406
Fantacci S, De Angelis F, Selloni A (2003) J Am Chem Soc 125:4381–4387
Angelis FD, Fantacci S, Selloni A, Nazeeruddin MK (2005) Chem Phys Lett 415:115–120
Xu Y, Chen WK, Cao MJ, Liu SH, Li JQ, Philippopoulos AI, Falaras P (2006) Chem Phys 330:204–211
Kurashige Y, Nakajima T, Kurashige S, Hirao K, Nishikitani Y (2007) J Phys Chem A 111:5544–5548
Hara K, Sato T, Katoh R, Furube A, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H (2003) J Phys Chem B 107:597–606
Zhang X, Zhang JJ, Xia YY (2008) J Photochem Photobiol A Chem 194:167–172
Liu Z (2008) J Mol Struct Theochem 862:44–48
Alexander BD, Dines TJ, Longhurst RW (2008) Chem Phys 352:19–27
Lee C, Sohlberg K (2010) Chem Phys 367:7–19
Ma R, Guo P, Cui H, Zhang X, Nazeeruddin MK, Grätzel M (2009) J Phys Chem A 113:10119–10124
Gao Y, Sun S, Han K (2009) Spectrochim Acta A Mol Biomol Spectrosc 71:2016–2022
Kumar PS, Vasudevan K, Prakasam A, Geetha M, Anbarasan PM (2010) Spectrochim Acta A Mol Biomol Spectrosc 77:45–50
Xu J, Wang L, Liang G, Bai Z, Wang L, Xu W, Shen X (2011) Spectrochim Acta A Mol Biomol Spectrosc 78:287–293
Senthilkumar P, Anbarasan PM (2011) J Mol Model 17:49–58
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, M.A. Robb, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima Y, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox IE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian Inc, Wallingford, CT
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Gorelsky SI (2010) University of Ottawa, Ottawa, Canada
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Howie WH, Claeyssens F, Miura H, Peter LM (2008) J Am Chem Soc 130:1367–1375
King BF, Weinhold F (1995) J Chem Phys 103:333–347
Lundqvist MJ, Nilsing M, Persson P, Lunell S (2006) Int J Quantum Chem 106:3214–3234
Bahers TL, Pauporté T, Scalmani G, Adamo C, Ciofini I (2009) Phys Chem Chem Phys 11:11276–11284
Watson DF, Meyer GJ (2005) Annu Rev Phys Chem 56:119–156
Boschloo G, Hagfeldt A (2005) J Phys Chem B 109:12093–12098
Zhang X, Zhang J-J, Xia Y-Y (2007) J Photochem Photobiol A Chem 185:283–288
Asbury JB, Hao E, Wang Y, Ghosh HN, Lian T (2001) J Phys Chem B 105:4545–4557
Acknowledgments
This work was supported by the Natural Science Foundation of China (No.51003082), and the Educational Commission of Hubei Province (Q20101606). The authors gratefully wish to express their thanks to the reviewers for critically reviewing the manuscript and making important suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Zhu, L., Wang, L. et al. The effect of anchoring group number on molecular structures and absorption spectra of triphenylamine sensitizers: a computational study. J Mol Model 18, 1767–1777 (2012). https://doi.org/10.1007/s00894-011-1208-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1208-z